Long-term potentiation

Inneuroscience,long-term potentiation(LTP) is a persistent strengthening ofsynapsesbased on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between twoneurons.[2]The opposite of LTP islong-term depression,which produces a long-lasting decrease in synaptic strength.
It is one of several phenomena underlyingsynaptic plasticity,the ability ofchemical synapsesto change their strength. As memories are thought to be encoded by modification ofsynaptic strength,[3]LTP is widely considered one of the major cellular mechanisms that underlieslearningandmemory.[2][3]
LTP was discovered in the rabbithippocampusbyTerje Lømoin 1966 and has remained a popular subject of research since. Many modern LTP studies seek to better understand its basic biology, while others aim to draw a causal link between LTP and behavioral learning. Still, others try to develop methods, pharmacologic or otherwise, of enhancing LTP to improve learning and memory. LTP is also a subject ofclinical research,for example, in the areas ofAlzheimer's diseaseandaddiction medicine.
History
Early theories of learning

At the end of the 19th century, scientists generally recognized that the number of neurons in the adult brain (roughly 100 billion[4]) did not increase significantly with age, giving neurobiologists good reason to believe that memories were generally not the result of new neuron production.[5]With this realization came the need to explain how memories could form in the absence of new neurons.
TheSpanishneuroanatomistSantiago Ramón y Cajalwas among the first to suggest a mechanism of learning that did not require the formation of new neurons. In his 1894Croonian Lecture,he proposed that memories might instead be formed by strengthening the connections between existing neurons to improve the effectiveness of their communication.[5]Hebbian theory,introduced byDonald Hebbin 1949, echoed Ramón y Cajal's ideas, further proposing that cells may grow new connections or undergo metabolic and synaptic changes that enhance their ability to communicate and create a neural network of experiences:[6]
Let us assume that the persistence or repetition of a reverberatory activity (or "trace" ) tends to induce lasting cellular changes that add to its stability.... When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A's efficiency, as one of the cells firing B, is increased.[7]
Eric Kandel(1964) and associates were some of the first researchers to discover long-term potentiation during their work with sea slug Aplysia. They attempted to apply behavioral conditioning to different cells in the slug’s neural network. Their results showed synaptic strength changes and researchers suggested that this may be due to a basic form of learning occurring within the slug.[8][9]
Though these theories of memory formation are now well established, they were farsighted for their time: late 19th and early 20th century neuroscientists and psychologists were not equipped with theneurophysiologicaltechniques necessary for elucidating the biological underpinnings of learning in animals. These skills would not come until the later half of the 20th century, at about the same time as the discovery of long-term potentiation.
Discovery
LTP was first observed byTerje Lømoin 1966 in theOslo,Norway,laboratory ofPer Andersen.[10][11]There, Lømo conducted a series ofneurophysiologicalexperiments onanesthetizedrabbits to explore the role of the hippocampus inshort-term memory.
Lømo's experiments focused on connections, or synapses, from theperforant pathwayto thedentate gyrus.These experiments were carried out by stimulating presynaptic fibers of the perforant pathway and recording responses from a collection of postsynaptic cells of the dentate gyrus. As expected, a single pulse of electrical stimulation to fibers of the perforant pathway causedexcitatory postsynaptic potentials(EPSPs) in cells of the dentate gyrus. What Lømo unexpectedly observed was that the postsynaptic cells' response to these single-pulse stimuli could be enhanced for a long period of time if he first delivered ahigh-frequency train of stimulito the presynaptic fibers. When such a train of stimuli was applied, subsequent single-pulse stimuli elicited stronger, prolonged EPSPs in the postsynaptic cell population. This phenomenon, whereby a high-frequency stimulus could produce a long-lived enhancement in the postsynaptic cells' response to subsequent single-pulse stimuli, was initially called "long-lasting potentiation".[12][13]
Timothy Bliss,who joined the Andersen laboratory in 1968,[10]collaborated with Lømo and in 1973 the two published the first characterization of long-lasting potentiation in therabbithippocampus.[12]Bliss and Tony Gardner-Medwin published a similar report of long-lasting potentiation in the awake animal which appeared in the same issue as the Bliss and Lømo report.[13]In 1975, Douglas and Goddard proposed "long-term potentiation" as a new name for the phenomenon of long-lasting potentiation.[14][15]Andersen suggested that the authors chose "long-term potentiation" perhaps because of its easily pronounced acronym, "LTP".[16]
Models and theory

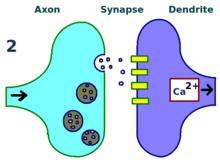
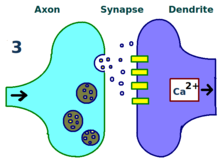

The physical and biological mechanism of LTP is still not understood, but some successful models have been developed.[1]Studies ofdendritic spines,protruding structures on dendrites that physically grow and retract over the course of minutes or hours, have suggested a relationship between theelectrical resistanceof the spine and the effective synapse strength, due to their relationship with intracellular calcium transients. Mathematical models such asBCM Theory,which depends also on intracellular calcium in relation toNMDA receptorvoltage gates,have been developed since the 1980s and modify the traditionala prioriHebbian learningmodel with both biological and experimental justification. Still, others have proposed re-arranging or synchronizing the relationship between receptor regulation, LTP, and synaptic strength.[17]
Types
Since its original discovery in the rabbit hippocampus, LTP has been observed in a variety of other neural structures, including thecerebral cortex,[18]cerebellum,[19]amygdala,[20]and many others.Robert Malenka,a prominent LTP researcher, has suggested that LTP may even occur at all excitatory synapses in the mammalian brain.[21]
Different areas of the brain exhibit different forms of LTP. The specific type of LTP exhibited between neurons depends on a number of factors. One such factor is the age of the organism when LTP is observed. For example, the molecular mechanisms of LTP in the immature hippocampus differ from those mechanisms that underlie LTP of the adult hippocampus.[22]The signalling pathways used by a particular cell also contribute to the specific type of LTP present. For example, some types of hippocampal LTP depend on theNMDA receptor,others may depend upon themetabotropic glutamate receptor(mGluR), while still others depend upon another molecule altogether.[21]The variety of signaling pathways that contribute to LTP and the wide distribution of these various pathways in the brain are reasons that the type of LTP exhibited between neurons depends only in part upon the anatomic location in which LTP is observed. For example, LTP in theSchaffer collateralpathway of the hippocampus is NMDA receptor-dependent - this was proved by the application ofAP5,an antagonist to the NMDA receptor, which prevented LTP in this pathway.[23]Conversely, LTP in themossy fiberpathway is NMDA receptor-independent, even though both pathways are in the hippocampus.[24]
The pre- and postsynaptic activity required to induce LTP are other criteria by which LTP is classified. Broadly, this allows classification of LTP into Hebbian, non-Hebbian, and anti-Hebbian mechanisms. Borrowing its name fromHebb's postulate,summarized by the maxim that "cells that fire together wire together,"Hebbian LTPrequires simultaneous pre- and postsynaptic depolarization for its induction.[25]Non-Hebbian LTPis a type of LTP that does not require such simultaneous depolarization of pre- and postsynaptic cells; an example of this occurs in the mossy fiber hippocampal pathway.[26]A special case of non-Hebbian LTP,anti-Hebbian LTPexplicitly requires simultaneous presynaptic depolarization and relative postsynaptic hyperpolarization for its induction.[27]
Owing to its predictable organization and readily inducible LTP, the CA1 hippocampus has become the prototypical site of mammalian LTP study. In particular, NMDA receptor-dependent LTP in the adult CA1 hippocampus is the most widely studied type of LTP,[21]and is therefore, the focus of this article.
Properties
NMDA receptor-dependent LTP exhibits several properties, including input specificity, associativity, cooperativity, and persistence.
- Input specificity
- Once induced, LTP at one synapse does not spread to other synapses; rather LTP isinput specific.Long-term potentiation is only propagated to those synapses according to the rules of associativity and cooperativity. However, the input specificity of LTP may be incomplete at short distances.[citation needed]One model to explain the input specificity of LTP was presented by Frey and Morris in 1997 and is called thesynaptic tagging and capturehypothesis.[28]
- Associativity
- Associativityrefers to the observation that when weak stimulation of a single pathway is insufficient for the induction of LTP, simultaneous strong stimulation of another pathway will induce LTP at both pathways.[29]
- Cooperativity
- LTP can be induced either by strongtetanic stimulationof a single pathway to a synapse, orcooperativelyvia the weaker stimulation of many. When one pathway into a synapse is stimulated weakly, it produces insufficient postsynaptic depolarization to induce LTP. In contrast, when weak stimuli are applied to many pathways that converge on a single patch of postsynaptic membrane, the individual postsynaptic depolarizations generated may collectively depolarize the postsynaptic cell enough to induce LTP cooperatively. Synaptic tagging, discussed later, may be a common mechanism underlying associativity and cooperativity.Bruce McNaughtonargues that any difference between associativity and cooperativity is strictly semantic.[30]Experiments performed by stimulating an array of individual dendritic spines, have shown that synaptic cooperativity by as few as two adjacent dendritic spines preventslong term depression(LTD) allowing only LTP.[31]
- Persistence
- LTP ispersistent,lasting from several minutes to many months, and it is this persistence that separates LTP from other forms ofsynaptic plasticity.[32]
Early phase
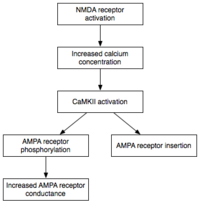

Maintenance
While induction entails thetransientactivation ofCaMKIIandPKC,maintenance of E-LTP (early-form LTP) is characterized by theirpersistentactivation. During this stagePKMzeta(PKMζ) which does not have dependence on calcium, become autonomously active. Consequently, they are able to carry out the phosphorylation events that underlie E-LTP expression.[34]
Expression
Phosphorylationis a chemical reaction in which a smallphosphategroup is added to another molecule to change that molecule's activity. Autonomously active CaMKII and PKC use phosphorylation to carry out the two major mechanisms underlying the expression of E-LTP. First, and most importantly, they phosphorylate existingAMPA receptorsto increase their activity.[21]Second, they mediate or modulate the insertion of additional AMPA receptors into the postsynaptic membrane.[21]Importantly, the delivery of AMPA receptors to the synapse during E-LTP is independent ofprotein synthesis.This is achieved by having a nonsynaptic pool of AMPA receptors adjacent to the postsynaptic membrane. When the appropriate LTP-inducing stimulus arrives, nonsynaptic AMPA receptors are rapidly trafficked into the postsynaptic membrane under the influence of protein kinases.[35]As mentioned previously, AMPA receptors are the brain's most abundant glutamate receptors and mediate the majority of its excitatory activity. By increasing the efficiency and number of AMPA receptors at the synapse, future excitatory stimuli generate larger postsynaptic responses.
While the above model of E-LTP describes entirely postsynaptic mechanisms for induction, maintenance, and expression, an additional component of expression may occur presynaptically.[36]One hypothesis of this presynaptic facilitation is that persistent CaMKII activity in the postsynaptic cell during E-LTP may lead to the synthesis of a "retrograde messenger", discussed later. According to this hypothesis, the newly synthesized messenger travels across the synaptic cleft from the postsynaptic to the presynaptic cell, leading to a chain of events that facilitate the presynaptic response to subsequent stimuli. Such events may include an increase in neurotransmitter vesicle number, probability of vesicle release, or both. In addition to the retrograde messenger underlying presynaptic expression inearly LTP,the retrograde messenger may also play a role in the expression of late LTP.
Late phase
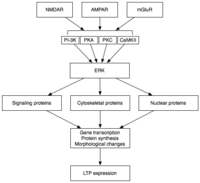
Late LTP (L-LTP) is the natural extension of E-LTP. Unlike E-LTP, which is independent of protein synthesis, L-LTP requiresgene transcription[37]andprotein synthesis[38]in the postsynaptic cell. Two phases of L-LTP exist: the first depends upon protein synthesis, while the second depends upon both gene transcription and protein synthesis.[33]These phases are occasionally called LTP2 and LTP3, respectively, with E-LTP referred to as LTP1 under this nomenclature.
Induction
Late LTP is induced by changes ingene expressionandprotein synthesisbrought about by the persistent activation of protein kinases activated during E-LTP, such as MAPK.[33][34][39]In fact, MAPK—specifically theextracellular signal-regulated kinase(ERK) subfamily of MAPKs—may be the molecular link between E-LTP and L-LTP, since many signaling cascades involved in E-LTP, including CaMKII and PKC, can converge on ERK.[39]Recent research has shown that the induction of L-LTP can depend on coincident molecular events, namely PKA activation and calcium influx, that converge on CRTC1 (TORC1), a potent transcriptional coactivator forcAMP response element binding protein(CREB).[40]This requirement for a molecular coincidence accounts perfectly for the associative nature of LTP, and, presumably, for that of learning.
Maintenance
Upon activation, ERK may phosphorylate a number of cytoplasmic and nuclear molecules that ultimately result in the protein synthesis and morphological changes observed in L-LTP.[33]These cytoplasmic and nuclear molecules may includetranscription factorssuch as CREB.[34]ERK-mediated changes in transcription factor activity may trigger the synthesis of proteins that underlie the maintenance of L-LTP. One such molecule may beprotein kinase Mζ(PKMζ), a persistently active kinase whose synthesis increases following LTP induction.[41][42]PKMζ is an atypical isoform of PKC that lacks a regulatory subunit and thus remains constitutively active.[41]Unlike other kinases that mediate LTP, PKMζ is active not just in the first 30 minutes following LTP induction; rather, PKMζ becomes a requirement for LTP maintenance only during the late phase of LTP.[41]PKMζ thus appears important for the persistence of memory and would be expected to be important in the maintenance oflong-term memory.Indeed, administration of a PKMζ inhibitor into the hippocampus of the rat results inretrograde amnesiawith intactshort-term memory;PKMζ does not play a role in the establishment of short-term memory.[42]PKMζ has recently been shown to underlie L-LTP maintenance[41][42]by directing the trafficking and reorganization of proteins in the synaptic scaffolding that underlie the expression of L-LTP.[41]Even more recently, transgenic mice lacking PKMζ demonstrate normal LTP, questioning the necessity of PKMζ.[43]
The long-term stabilization of synaptic changes is also determined by a parallel increase of pre- and postsynaptic structures such asaxonal bouton,dendritic spineandpostsynaptic density.[44] On the molecular level, an increase of the postsynaptic scaffolding proteinsPSD-95andHomer1chas been shown to correlate with the stabilization of synaptic enlargement.[44]
Expression
The identities of only a few proteins synthesized during L-LTP are known. Regardless of their identities, it is thought that they contribute to the increase indendritic spinenumber, surface area, and postsynaptic sensitivity to neurotransmitter associated with L-LTP expression.[33]The latter may be brought about in part by the enhanced synthesis of AMPA receptors during L-LTP.[33]Late LTP is also associated with the presynaptic synthesis ofsynaptotagminand an increase insynaptic vesiclenumber, suggesting that L-LTP induces protein synthesis not only in postsynaptic cells, but in presynaptic cells as well.[33]As mentioned previously, for postsynaptic LTP induction to result in presynaptic protein synthesis, there must be communication from the postsynaptic to the presynaptic cell. This may occur via the synthesis of a retrograde messenger, discussed later.
Even in studies restricted to postsynaptic events, investigators have not determined the location of the protein synthesis that underlies L-LTP. Specifically, it is unclear whether protein synthesis takes place in the postsynapticcell bodyor in itsdendrites.[39]Despite having observedribosomes(the major components of the protein synthesis machinery) in dendrites as early as the 1960s, prevailing wisdom was that the cell body was the predominant site of protein synthesis in neurons.[39]This reasoning was not seriously challenged until the 1980s, when investigators reported observing protein synthesis in dendrites whose connection to their cell body had been severed.[39]More recently, investigators have demonstrated that this type of local protein synthesis is necessary for some types of LTP.[45][46]
One reason for the popularity of the local protein synthesis hypothesis is that it provides a possible mechanism for the specificity associated with LTP.[39]Specifically, if indeed local protein synthesis underlies L-LTP, only dendritic spines receiving LTP-inducing stimuli will undergo LTP; the potentiation will not be propagated to adjacent synapses. By contrast, global protein synthesis that occurs in the cell body requires that proteins be shipped out to every area of the cell, including synapses that have not received LTP-inducing stimuli. Whereas local protein synthesis provides a mechanism for specificity, global protein synthesis would seem to directly compromise it. However, as discussed later, the synaptic tagging hypothesis successfully reconciles global protein synthesis, synapse specificity, and associativity.
Retrograde signaling
Retrograde signaling is a hypothesis that attempts to explain that, while LTP is induced and expressed postsynaptically, some evidence suggests that it is expressed presynaptically as well.[21][36][47]The hypothesis gets its name because normalsynaptic transmissionis directional and proceeds from the presynaptic to the postsynaptic cell. For induction to occur postsynaptically and be partially expressed presynaptically, a message must travel from the postsynaptic cell to the presynaptic cell in aretrograde(reverse) direction. Once there, the message presumably initiates a cascade of events that leads to a presynaptic component of expression, such as the increased probability ofneurotransmitter vesiclerelease.[48]
Retrograde signaling is currently a contentious subject as some investigators do not believe the presynaptic cell contributes at all to the expression of LTP.[21]Even among proponents of the hypothesis there is controversy over the identity of the messenger. Early thoughts focused onnitric oxide,while most recent evidence points tocell adhesionproteins.[21]
Synaptic tagging
Before the local protein synthesis hypothesis gained significant support, there was general agreement that the protein synthesis underlying L-LTP occurred in the cell body. Further, there was thought that the products of this synthesis were shipped cell-wide in a nonspecific manner. It thus became necessary to explain how protein synthesis could occur in the cell body without compromising LTP's input specificity. The synaptic tagging hypothesis attempts to solve the cell's difficult problem of synthesizing proteins in the cell body but ensuring they only reach synapses that have received LTP-inducing stimuli.
The synaptic tagging hypothesis proposes that a "synaptic tag" is synthesized at synapses that have received LTP-inducing stimuli, and that this synaptic tag may serve to capture plasticity-related proteins shipped cell-wide from the cell body.[49]Studies of LTP in themarine snailAplysia californicahave implicated synaptic tagging as a mechanism for the input-specificity of LTP.[50][51]There is some evidence that given two widely separated synapses, an LTP-inducing stimulus at one synapse drives several signaling cascades (described previously) that initiates gene expression in the cell nucleus. At the same synapse (but not the unstimulated synapse), local protein synthesis creates a short-lived (less than three hours) synaptic tag. The products of gene expression are shipped globally throughout the cell, but are only captured by synapses that express the synaptic tag. Thus only the synapse receiving LTP-inducing stimuli is potentiated, demonstrating LTP's input specificity.
The synaptic tag hypothesis may also account for LTP's associativity and cooperativity. Associativity (seeProperties) is observed when one synapse is excited with LTP-inducing stimulation while a separate synapse is only weakly stimulated. Whereas one might expect only the strongly stimulated synapse to undergo LTP (since weak stimulation alone is insufficient to induce LTP at either synapse),bothsynapses will in fact undergo LTP. While weak stimuli are unable to induce protein synthesis in the cell body, they may prompt the synthesis of a synaptic tag. Simultaneous strong stimulation of a separate pathway, capable of inducing cell body protein synthesis, then may prompt the production of plasticity-related proteins, which are shipped cell-wide. With both synapses expressing the synaptic tag, both would capture the protein products resulting in the expression of LTP in both the strongly stimulated and weakly stimulated pathways.
Cooperativity is observed when two synapses are activated by weak stimuli incapable of inducing LTP when stimulated individually. But upon simultaneous weak stimulation, both synapses undergo LTP in a cooperative fashion. Synaptic tagging does not explain how multiple weak stimuli can result in a collective stimulus sufficient to induce LTP (this is explained by the postsynaptic summation of EPSPs described previously). Rather, synaptic tagging explains the ability of weakly stimulated synapses, none of which are capable of independently generating LTP, to receive the products of protein synthesis initiated collectively. As before, this may be accomplished through the synthesis of a local synaptic tag following weak synaptic stimulation.
Modulation
| Modulator | Target |
|---|---|
| β-Adrenergic receptor | cAMP, MAPK amplification |
| Nitric oxide synthase | Guanylyl cyclase, PKG, NMDAR |
| Dopamine receptor | cAMP, MAPK amplification |
| Metabotropic glutamate receptor | PKC, MAPK amplification |
As described previously, the molecules that underlie LTP can be classified as mediators or modulators. A mediator of LTP is a molecule, such as the NMDA receptor or calcium, whose presence and activity is necessary for generating LTP under nearly all conditions. By contrast, a modulator is a molecule that can alter LTP but is not essential for its generation or expression.[21]
In addition to the signaling pathways described above, hippocampal LTP may be altered by a variety of modulators. For example, thesteroid hormoneestradiolmay enhance LTP by driving CREB phosphorylation and subsequentdendritic spinegrowth.[52]Additionally,β-adrenergic receptoragonists such asnorepinephrinemay alter the protein synthesis-dependent late phase of LTP.[53]Nitric oxide synthaseactivity may also result in the subsequent activation of guanylyl cyclase and PKG.[54]Similarly, activation ofdopamine receptorsmay enhance LTP through the cAMP/PKA signaling pathway.[55][56]
Relationship to behavioral memory
While the long-term potentiation of synapses in cell culture seems to provide an elegant substrate for learning and memory, the contribution of LTP to behavioral learning — that is, learning at the level of the whole organism — cannot simply be extrapolated fromin vitrostudies. For this reason, considerable effort has been dedicated to establishing whether LTP is a requirement for learning and memory in living animals. Because of this, LTP also plays a crucial role infear processing.
Spatial memory

In 1986, Richard Morris provided some of the first evidence that LTP was indeed required for the formation of memoriesin vivo.[57]He tested thespatial memoryof rats by pharmacologically modifying their hippocampus, a brain structure whose role in spatial learning is well established. Rats were trained on theMorris water maze,a spatial memory task in which rats swim in a pool of murky water until they locate the platform hidden beneath its surface. During this exercise, normal rats are expected to associate the location of the hidden platform with salient cues placed at specific positions around the circumference of the maze. After training, one group of rats had their hippocampi bathed in the NMDA receptor blockerAPV,while the other group served as thecontrol.Both groups were then subjected to the water maze spatial memory task. Rats in the control group were able to locate the platform and escape from the pool, while the performance of APV-treated rats was significantly impaired. Moreover, when slices of the hippocampus were taken from both groups, LTP was easily induced in controls, but could not be induced in the brains of APV-treated rats. This provided early evidence that the NMDA receptor — and by extension, LTP — was required for at least some types of learning and memory.
Similarly,Susumu Tonegawademonstrated in 1996 that the CA1 area of the hippocampus is crucial to the formation of spatial memories in living mice.[58]So-calledplace cellslocated in this region become active only when the rat is in a particular location — called aplace field— in the environment. Since these place fields are distributed throughout the environment, one interpretation is that groups of place cells form maps in the hippocampus. The accuracy of these maps determines how well a rat learns about its environment and thus how well it can navigate it. Tonegawa found that by impairing the NMDA receptor, specifically by genetically removing the NR1 subunit in the CA1 region, the place fields generated were substantially less specific than those of controls. That is, mice produced faulty spatial maps when their NMDA receptors were impaired. As expected, these mice performed very poorly on spatial tasks compared to controls, further supporting the role of LTP in spatial learning.
Enhanced NMDA receptor activity in the hippocampus has also been shown to produce enhanced LTP and an overall improvement in spatial learning. In 1999, Tanget al.produced a line of mice with enhanced NMDA receptor function by overexpressing theNR2Bsubunit in the hippocampus.[59][60]The resulting smart mice, nicknamed "Doogie mice" after the fictional prodigious doctorDoogie Howser,had larger LTP and excelled at spatial learning tasks, reinforcing LTP's importance in the formation of hippocampus-dependent memories.
Inhibitory avoidance
In 2006,Jonathan Whitlockand colleagues reported on a series of experiments that provided perhaps the strongest evidence of LTP's role in behavioral memory, arguing that to conclude that LTP underlies behavioral learning, the two processes must both mimic and occlude one another.[61]Employing an inhibitory avoidance learning paradigm, researchers trained rats in a two-chambered apparatus with light and dark chambers, the latter being fitted with a device that delivered a foot shock to the rat upon entry. An analysis of CA1 hippocampal synapses revealed that inhibitory avoidance training inducedin vivoAMPA receptor phosphorylation of the same type as that seen in LTPin vitro;that is, inhibitory avoidance training mimicked LTP. In addition, synapses potentiated during training could not be further potentiated by experimental manipulations that would have otherwise induced LTP; that is, inhibitory avoidance training occluded LTP. In a response to the article, Timothy Bliss and colleagues remarked that these and related experiments "substantially advance the case for LTP as a neural mechanism for memory."[62]
Clinical significance
The role of LTP in disease is less clear than its role in basic mechanisms ofsynaptic plasticity.However, alterations in LTP may contribute to a number ofneurological diseases,includingdepression,Parkinson's disease,epilepsy,andneuropathic pain.[63]Impaired LTP may also have a role inAlzheimer's diseaseanddrug addiction.
Alzheimer's disease

LTP has received much attention among those who studyAlzheimer's disease(AD), aneurodegenerative diseasethat causes marked cognitive decline anddementia.Much of this deterioration occurs in association with degenerative changes in the hippocampus and othermedial temporal lobestructures. Because of the hippocampus' well established role in LTP, some have suggested that the cognitive decline seen in individuals with AD may result from impaired LTP.
In a 2003 review of the literature, Rowanet al.proposed one model for how LTP might be affected in AD.[64]AD appears to result, at least in part, from misprocessing ofamyloid precursor protein(APP). The result of this abnormal processing is the accumulation of fragments of this protein, calledamyloid β(Aβ). Aβ exists in both soluble and fibrillar forms. Misprocessing of APP results in the accumulation of soluble Aβ that, according to Rowan's hypothesis, impairs hippocampal LTP and may lead to the cognitive decline seen early in AD.
AD may also impair LTP through mechanisms distinct from Aβ. For example, one study demonstrated that the enzyme PKMζ accumulates inneurofibrillary tangles,which are a pathologic marker of AD. PKMζ is an enzyme with critical importance in themaintenance of late LTP.[65]
Drug addiction
Research in the field ofaddiction medicinehas also recently[when?]turned its focus to LTP, owing to the hypothesis thatdrug addictionrepresents a powerful form of learning and memory.[66]Addiction is a complex neurobehavioral phenomenon involving various parts of the brain, such as theventral tegmental area(VTA) andnucleus accumbens(NAc). Studies have demonstrated that VTA and NAc synapses are capable of undergoing LTP[66]and that this LTP may be responsible for the behaviors that characterize addiction.[67]
See also
- Neuroplasticity
- Actin remodeling of neurons
- Transcranial direct-current stimulation
- Post-tetanic potentiation
References
- ^Paradiso MA, Bear MF, Connors BW (2007).Neuroscience: Exploring the Brain.Hagerstwon, MD: Lippincott Williams & Wilkins. p.718.ISBN978-0-7817-6003-4.
- ^abCooke SF, Bliss TV (July 2006)."Plasticity in the human central nervous system".Brain.129(Pt 7): 1659–73.doi:10.1093/brain/awl082.PMID16672292.
- ^abBliss TV, Collingridge GL (January 1993). "A synaptic model of memory: long-term potentiation in the hippocampus".Nature.361(6407): 31–9.Bibcode:1993Natur.361...31B.doi:10.1038/361031a0.PMID8421494.S2CID4326182.
- ^Williams RW, Herrup K (1988). "The control of neuron number".Annual Review of Neuroscience.11(1): 423–53.doi:10.1146/annurev.ne.11.030188.002231.PMID3284447.
- ^abSantiago RC (1894)."The Croonian Lecture: La Fine Structure des Centres Nerveux".Proceedings of the Royal Society of London.55(331–335): 444–468.Bibcode:1894RSPS...55..444C.doi:10.1098/rspl.1894.0063.
- ^Hebb D (1949).The Organization of Behavior: A NEUROPSYCHOLOGICAL THEORY.New York: JOHN WILEY if SONS, Inc.ISBN978-0805843002.
- ^Hebb DO (1949).Organization of Behavior: a Neuropsychological Theory.New York: John Wiley.ISBN978-0-471-36727-7.
- ^Kandel ER, Tauc L (November 1965)."Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans".The Journal of Physiology.181(1): 1–27.doi:10.1113/jphysiol.1965.sp007742.PMC1357435.PMID5866283.
- ^Patihis L (October 2018)."The historical significance of the discovery of long-term potentiation: an overview and evaluation for nonexperts".American Journal of Psychology.131(3): 369–80.doi:10.5406/amerjpsyc.131.3.0369.
- ^abLømo T (April 2003)."The discovery of long-term potentiation".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.358(1432): 617–20.doi:10.1098/rstb.2002.1226.PMC1693150.PMID12740104.
- ^Lømo T (1966). "Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation".Acta Physiologica Scandinavica.68(Suppl 277): 128.
- ^abBliss TV, Lomo T (July 1973)."Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path".The Journal of Physiology.232(2): 331–56.doi:10.1113/jphysiol.1973.sp010273.PMC1350458.PMID4727084.
- ^abBliss TV, Gardner-Medwin AR (July 1973)."Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path".The Journal of Physiology.232(2): 357–74.doi:10.1113/jphysiol.1973.sp010274.PMC1350459.PMID4727085.
- ^While the term "long term potentiation" appeared once in the original Bliss and Lømo paper, it was not formally proposed for the phenomenon until the Douglas and Goddard paper.
- ^Douglas RM, Goddard GV (March 1975). "Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus".Brain Research.86(2): 205–15.doi:10.1016/0006-8993(75)90697-6.PMID163667.S2CID43260928.
- ^Andersen P (April 2003)."A prelude to long-term potentiation".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.358(1432): 613–5.doi:10.1098/rstb.2002.1232.PMC1693144.PMID12740103.
- ^McEachern JC, Shaw CA (June 1996). "An alternative to the LTP orthodoxy: a plasticity-pathology continuum model".Brain Research. Brain Research Reviews.22(1): 51–92.doi:10.1016/0165-0173(96)00006-9.PMID8871785.S2CID41680613.8871785.
- ^Bear MF (1996)."A synaptic basis for memory storage in the cerebral cortex".Proceedings of the National Academy of Sciences.93(24): 13453–13459.Bibcode:1996PNAS...9313453B.doi:10.1073/pnas.93.24.13453.PMC33630.PMID8942956.
- ^Ouardouz M, Sastry BR (2000). "Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei".Journal of Neurophysiology.84(3): 1414–1421.doi:10.1152/jn.2000.84.3.1414.PMID10980014.S2CID16972473.
- ^Clugnet MC, LeDoux JE (August 1990)."Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body".The Journal of Neuroscience.10(8): 2818–24.doi:10.1523/JNEUROSCI.10-08-02818.1990.PMC6570263.PMID2388089.
- ^abcdefghiMalenka RC, Bear MF (September 2004)."LTP and LTD: an embarrassment of riches".Neuron.44(1): 5–21.doi:10.1016/j.neuron.2004.09.012.PMID15450156.S2CID79844.
- ^Yasuda H, Barth AL, Stellwagen D, Malenka RC (January 2003). "A developmental switch in the signaling cascades for LTP induction".Nature Neuroscience.6(1): 15–6.doi:10.1038/nn985.PMID12469130.S2CID28913342.
- ^Collingridge GL, Kehl SJ, McLennan H (January 1983)."Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus".The Journal of Physiology.334:33–46.doi:10.1113/jphysiol.1983.sp014478.PMC1197298.PMID6306230.
- ^Harris EW, Cotman CW (September 1986). "Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists".Neuroscience Letters.70(1): 132–7.doi:10.1016/0304-3940(86)90451-9.PMID3022192.S2CID42647125.
- ^Wigström H, Gustafsson B (1986). "Postsynaptic control of hippocampal long-term potentiation".Journal de Physiologie.81(4): 228–36.PMID2883309.
- ^Urban NN, Barrionuevo G (July 1996)."Induction of hebbian and non-hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation".The Journal of Neuroscience.16(13): 4293–9.doi:10.1523/JNEUROSCI.16-13-04293.1996.PMC6579001.PMID8753890.
- ^Kullmann DM, Lamsa K (March 2008)."Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation".The Journal of Physiology.586(6): 1481–6.doi:10.1113/jphysiol.2007.148064.PMC2375711.PMID18187472.
- ^Frey U, Morris RG (February 1997). "Synaptic tagging and long-term potentiation".Nature.385(6616): 533–536.Bibcode:1997Natur.385..533F.doi:10.1038/385533a0.PMID9020359.S2CID4339789.
- ^Hao L, Yang Z, Lei J (1 May 2018)."Underlying Mechanisms of Cooperativity, Input Specificity, and Associativity of Long-Term Potentiation Through a Positive Feedback of Local Protein Synthesis".Frontiers in Computational Neuroscience.12:25.doi:10.3389/fncom.2018.00025.PMC5938377.PMID29765314.
- ^McNaughton BL (April 2003)."Long-term potentiation, cooperativity and Hebb's cell assemblies: a personal history".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.358(1432): 629–34.doi:10.1098/rstb.2002.1231.PMC1693161.PMID12740107.
- ^Tazerart S, Mitchell DE, Miranda-Rottmann S, Araya R (August 2020)."A spike-timing-dependent plasticity rule for dendritic spines".Nature Communications.11(1): 4276.Bibcode:2020NatCo..11.4276T.doi:10.1038/s41467-020-17861-7.PMC7449969.PMID32848151.
- ^Abraham WC (April 2003)."How long will long-term potentiation last?".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.358(1432): 735–44.doi:10.1098/rstb.2002.1222.PMC1693170.PMID12740120.
- ^abcdefghLynch MA (January 2004)."Long-term potentiation and memory".Physiological Reviews.84(1): 87–136.doi:10.1152/physrev.00014.2003.PMID14715912.
- ^abcdSweatt JD (1999)."Toward a molecular explanation for long-term potentiation".Learning & Memory.6(5): 399–416.doi:10.1101/lm.6.5.399.PMID10541462.
- ^Malinow R (April 2003)."AMPA receptor trafficking and long-term potentiation".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.358(1432): 707–14.doi:10.1098/rstb.2002.1233.PMC1693162.PMID12740116.
- ^abEmptage NJ, Reid CA, Fine A, Bliss TV (June 2003)."Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer-associational synapses".Neuron.38(5): 797–804.doi:10.1016/S0896-6273(03)00325-8.PMID12797963.S2CID13629691.
- ^Frey U, Frey S, Schollmeier F, Krug M (February 1996)."Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro".The Journal of Physiology.490.490(Pt 3): 703–11.doi:10.1113/jphysiol.1996.sp021179.PMC1158708.PMID8683469.
- ^Frey U, Krug M, Reymann KG, Matthies H (June 1988). "Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro".Brain Research.452(1–2): 57–65.doi:10.1016/0006-8993(88)90008-X.PMID3401749.S2CID39245231.
- ^abcdefKelleher RJ, Govindarajan A, Tonegawa S (September 2004)."Translational regulatory mechanisms in persistent forms of synaptic plasticity".Neuron.44(1): 59–73.doi:10.1016/j.neuron.2004.09.013.PMID15450160.S2CID1511103.
- ^Kovács KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, et al. (March 2007)."TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity".Proceedings of the National Academy of Sciences of the United States of America.104(11): 4700–5.Bibcode:2007PNAS..104.4700K.doi:10.1073/pnas.0607524104.PMC1838663.PMID17360587.
- ^abcdeSerrano P, Yao Y, Sacktor TC (February 2005)."Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation".The Journal of Neuroscience.25(8): 1979–84.doi:10.1523/JNEUROSCI.5132-04.2005.PMC6726070.PMID15728837.
- ^abcPastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (August 2006). "Storage of spatial information by the maintenance mechanism of LTP".Science.313(5790): 1141–4.Bibcode:2006Sci...313.1141P.CiteSeerX10.1.1.453.2136.doi:10.1126/science.1128657.PMID16931766.S2CID7260010.
- ^Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL (January 2013)."PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory".Nature.493(7432): 420–3.Bibcode:2013Natur.493..420V.doi:10.1038/nature11802.PMC3830948.PMID23283174.
- ^abMeyer D, Bonhoeffer T, Scheuss V (April 2014)."Balance and stability of synaptic structures during synaptic plasticity".Neuron.82(2): 430–43.doi:10.1016/j.neuron.2014.02.031.PMID24742464.
- ^Kang H, Schuman EM (September 1996). "A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity".Science.273(5280): 1402–6.Bibcode:1996Sci...273.1402K.doi:10.1126/science.273.5280.1402.PMID8703078.S2CID38648558.
- ^Steward O, Worley PF (June 2001)."A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites".Proceedings of the National Academy of Sciences of the United States of America.98(13): 7062–8.Bibcode:2001PNAS...98.7062S.doi:10.1073/pnas.131146398.PMC34623.PMID11416188.
- ^Pavlidis P,Montgomery J,Madison DV (June 2000)."Presynaptic protein kinase activity supports long-term potentiation at synapses between individual hippocampal neurons".The Journal of Neuroscience.20(12): 4497–505.doi:10.1523/JNEUROSCI.20-12-04497.2000.PMC6772468.PMID10844019.
- ^Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, et al. (September 2003)."Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses".Neuron.39(6): 975–90.doi:10.1016/S0896-6273(03)00543-9.PMID12971897.S2CID7117772.
- ^Frey U, Morris RG (February 1997). "Synaptic tagging and long-term potentiation".Nature.385(6616): 533–6.Bibcode:1997Natur.385..533F.doi:10.1038/385533a0.PMID9020359.S2CID4339789.
- ^Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, et al. (December 1997)."Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage".Cell.91(7): 927–38.doi:10.1016/S0092-8674(00)80484-5.PMID9428516.S2CID16423304.
- ^Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, et al. (October 1999)."A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis".Cell.99(2): 221–37.doi:10.1016/S0092-8674(00)81653-0.PMID10535740.
- ^Segal M, Murphy DD (1999)."CREB activation mediates plasticity in cultured hippocampal neurons".Neural Plasticity.6(3): 1–7.doi:10.1155/NP.1998.1.PMC2565317.PMID9920677.
- ^Straube T, Frey JU (2003). "Involvement of beta-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength".Neuroscience.119(2): 473–9.doi:10.1016/S0306-4522(03)00151-9.PMID12770561.S2CID23436714.
- ^Lu YF, Kandel ER, Hawkins RD (December 1999)."Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus".The Journal of Neuroscience.19(23): 10250–61.doi:10.1523/JNEUROSCI.19-23-10250.1999.PMC6782403.PMID10575022.
- ^Frey U, Matthies H, Reymann KG, Matthies H (August 1991). "The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro".Neuroscience Letters.129(1): 111–4.doi:10.1016/0304-3940(91)90732-9.PMID1833673.S2CID45084596.
- ^Otmakhova NA, Lisman JE (December 1996)."D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses".The Journal of Neuroscience.16(23): 7478–86.doi:10.1523/JNEUROSCI.16-23-07478.1996.PMC6579102.PMID8922403.
- ^Morris RG, Anderson E, Lynch GS, Baudry M (1986). "Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5".Nature.319(6056): 774–6.Bibcode:1986Natur.319..774M.doi:10.1038/319774a0.PMID2869411.S2CID4356601.
- ^McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA (December 1996)."Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice".Cell.87(7): 1339–49.doi:10.1016/S0092-8674(00)81828-0.PMID8980239.S2CID5131226.
- ^Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. (1999)."Genetic enhancement of learning and memory in mice".Nature.401(6748): 63–69.Bibcode:1999Natur.401...63T.doi:10.1038/43432.PMID10485705.S2CID481884.
- ^Tang Y, Wang H, Feng R, Kyin M, Tsien J (2001). "Differential effects of enrichment on learning and memory function in NR2B transgenic mice".Neuropharmacology.41(6): 779–90.doi:10.1016/S0028-3908(01)00122-8.PMID11640933.S2CID23602265.
- ^Whitlock JR, Heynen AJ, Shuler MG, Bear MF (August 2006). "Learning induces long-term potentiation in the hippocampus".Science.313(5790): 1093–7.Bibcode:2006Sci...313.1093W.doi:10.1126/science.1128134.PMID16931756.S2CID612352.
- ^Bliss TV, Collingridge GL, Laroche S (August 2006). "Neuroscience. ZAP and ZIP, a story to forget".Science.313(5790): 1058–9.doi:10.1126/science.1132538.PMID16931746.S2CID27735098.
- ^Cooke SF, Bliss TV (July 2006)."Plasticity in the human central nervous system".Brain.129(Pt 7): 1659–73.doi:10.1093/brain/awl082.PMID16672292.
- ^abRowan MJ, Klyubin I, Cullen WK, Anwyl R (April 2003)."Synaptic plasticity in animal models of early Alzheimer's disease".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.358(1432): 821–8.doi:10.1098/rstb.2002.1240.PMC1693153.PMID12740129.
- ^Crary JF, Shao CY, Mirra SS, Hernandez AI, Sacktor TC (April 2006). "Atypical protein kinase C in neurodegenerative disease I: PKMzeta aggregates with limbic neurofibrillary tangles and AMPA receptors in Alzheimer disease".Journal of Neuropathology and Experimental Neurology.65(4): 319–26.doi:10.1097/01.jnen.0000218442.07664.04.PMID16691113.
- ^abKauer JA, Malenka RC (November 2007)."Synaptic plasticity and addiction".Nature Reviews. Neuroscience.8(11): 844–58.doi:10.1038/nrn2234.PMID17948030.S2CID38811195.
- ^Wolf ME (August 2003)."LTP may trigger addiction".Molecular Interventions.3(5): 248–52.doi:10.1124/mi.3.5.248.PMID14993438.
Further reading
- Bliss T, Collingridge G, Morris R, eds. (2004).Long-term potentiation: enhancing neuroscience for 30 years.Oxford University Press.doi:10.1093/oso/9780198530305.001.0001.ISBN978-0-19-853030-5.
- Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J (2007)."10. Synaptic Plasticity in the Hippocampus".The Hippocampus Book.Oxford University Press. pp. 350–474.doi:10.1093/acprof:oso/9780195100273.003.0010.ISBN978-0-19-510027-3.
External links
- Researchers provide first evidence for learning mechanism,aPhysOrg.comreport on 2006 study by Bear and colleagues.
- Short video documentary about the Doogie mice.(RealPlayerformat)
- "Smart Mouse", a Quantum ABC TV episode about the Doogie mice.
- Long-Term+Potentiationat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
