MALT lymphoma
| MALT lymphoma | |
|---|---|
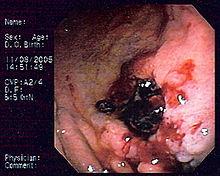 | |
| Endoscopic image of gastric MALT lymphoma taken in body ofstomachin patient who presented withupper GI hemorrhage.Appearance is similar togastric ulcerwith adherent clot. | |
| Specialty | Oncology |
MALT lymphoma(also calledMALToma) is a form oflymphomainvolving themucosa-associated lymphoid tissue(MALT), frequently of thestomach,but virtually any mucosal site can be affected. It is acanceroriginating fromB cellsin the marginal zone of the MALT.
Diagnosis and staging[edit]
MALT lymphoma is often a multifocal disease in the organ of origin and is frequently macroscopically indistinguishable from other disease processes in theGI tract.Endoscopyis key to diagnosing MALT lymphoma, with multiple biopsies of the visible lesions required, as well as samples of macroscopically normal tissue, termed gastric mapping. Histologically, there is expansion of the marginal zone compartment with development of sheets of neoplastic small lymphoid cells.[1]The morphology of the neoplastic cells is variable with small mature lymphocytes, cells resembling centrocytes (centrocyte like cells), or marginal zone/monocytoid B cells. Plasmacytoid or plasmacytic differentiation is frequent. Lymphoid follicles are ubiquitous to MALT lymphoma but may be indistinct as they are often overrun or colonized by the neoplastic cells. Large transformed B cells are presently scattered among the small cell population. If these large cells are present in clusters or sheets, a diagnosis of associated large B-cell lymphoma should be considered. A characteristic feature of MALT lymphoma is the presence of neoplastic cells within epithelial structures with associated destruction of the glandular architecture to form lymphoepithelial lesions.[2]
MALT lymphoma may be difficult to distinguish from reactive infiltrates, and in some cases, multiple endoscopies are required before a confident diagnosis is reached. The Wotherspoon score, which grades the presence of histological features associated with MALT lymphoma, is useful in expressing confidence in diagnosis at presentation. Immunohistochemistry can be used to help distinguish MALT lymphoma from other small B-cell NHLs. B-cell-associated antigens such as CD19, CD20, CD22, and CD79a are usually expressed. In contrast to small lymphocytic lymphoma and MCL, staining for CD5 is usually negative, and these lymphomas can be further distinguished with CD23 (positive in small lymphocytic lymphoma) and CyclinD1 (positive in MCL).[3]
Associations[edit]
Gastric MALT lymphoma is frequently associated (72–98%) with chronicinflammationas a result of the presence ofHelicobacter pylori,[4]potentially involving chronic inflammation, or the action ofH. pylorivirulence factors such asCagA.[5]
The initial diagnosis is made bybiopsyof suspicious lesions onesophagogastroduodenoscopy(EGD, upper endoscopy). Simultaneous tests forH. pyloriare also done to detect the presence of this microbe.[6]
In other sites, chronic immune stimulation is also suspected in the pathogenesis (e.g. association between chronicautoimmune diseasessuch asSjögren's syndromeandHashimoto's thyroiditis,and MALT lymphoma of thesalivary glandand thethyroid).[6]
Treatment[edit]
Owing to the causal relationship betweenH. pyloriinfection and gastric MALT lymphoma, identification of the infection is imperative. Histological examination of GI biopsies yields a sensitivity of 95% with five biopsies,[7]but these should be from sites uninvolved by lymphoma and the identification of the organism may be compromised by areas of extensive intestinal metaplasia. As proton-pump inhibition can suppress infection, any treatment with this class of drug should be ceased 2 weeks prior to biopsy retrieval. Serology should be performed if histology is negative, to detect suppressed or recently treated infections.[8]Following the recognition of the association of gastric MALT lymphoma withH. pyloriinfection, it was established that early-stage gastric disease could be cured byH. pylorieradication, which is now the mainstay of therapy. Fifty to 95% of cases achieve complete response (CR) withH. pyloritreatment.[9][10]
A t(11;18)(q21;q21)chromosomal translocation,giving rise to anAPI2-MLTfusion gene,[11]is predictive of poor response to eradication therapy.[12]
Radiotherapy[edit]
Radiotherapy is a valid first option forMALT lymphoma.It provides local control and potential cure in localized gastric stage IE and II 1E disease with 5-year EFS of 85-100% reported in retrospective studies.[13][14]However, the irradiation field is potentially large as it must include the whole stomach, which can vary greatly in size and shape. Irradiation techniques have improved considerably in the last 20 years, including treating the patient in a fasting state, decreasing the irradiated field and required dose. The moderate dose of 30 Gray (Gy) of involved-field radiotherapy administered in 15 fractions (doses) can be associated with tolerable toxicity and excellent outcomes. Hence, radiotherapy is the preferred approach for local disease where antibiotic therapy has failed, or is not indicated. Evidence also suggests that radiotherapy can be utilized to control localized relapses outside the original radiation field.[15]
Chemotherapy[edit]
MALT lymphomais exquisitely immunotherapy sensitive.Chemotherapyis reserved for those uncommon patients with disseminated disease at presentation or lack of response to local treatment. Rituximab, the anti-CD20 chimeric antibody, is a key component of therapy. Responses vary from 55% to 77% with monotherapy and 100% in combination withchemotherapy.[16][17]Ocular MALT lymphoma can be treated by intralesional administration of rituximab.[18]Oral alkylating agents such as cyclophosphamide or chlorambucil have been administered for a median duration of 12 months with high rates of disease control (CR up to 75%) but appear not to be active in t(11;18) disease.[19]The purine nucleoside analogs fludarabine and cladribine also demonstrate activity,[20]the latter conferring a CR rate of 84% (100% in those with gastric primaries) in a small study.[21]A pivotal study of rituximab plus chlorambucil compared with chlorambucil alone (IELSG-19 study, n = 227) demonstrated a significantly higher CR rate (78% vs. 65%; p = 0.017) and 5-year EFS (68% vs. 50%; p = 0.024) over chlorambucil alone. However, 5-year OS was not improved (88% in both arms). First-line treatment of choice is generally rituximab in combination with single alkylating agents or fludarabine, or a combination of all three drugs. The final results of this study, including the later addition of a rituximab-alone arm, are pending.[22]
Two othergeneticalterations are known:[citation needed]
- t(1;14)(p22;q32), which deregulatesBCL10,at the locus1p22.
- t(14;18)(q32;q21), which deregulatesMALT1,at the locus18q21.
These seem to turn on the same pathway as API2-MLT (i.e., that ofNF-κB). They both act uponIGH,[23]which is at the locus14q32.
Epidemiology[edit]
Of allcancers involving the same class of blood cell,8% of cases are MALT lymphomas.[24]
See also[edit]
References[edit]
- ^Taal BG, Boot H, van Heerde P, de Jong D, Hart AA, Burgers JM (October 1996)."Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept".Gut.39(4): 556–61.doi:10.1136/gut.39.4.556.PMC1383269.PMID8944565.
- ^Jankowski J, Hawk E, eds. (2012).Handbook of Gastrointestinal Cancer(2 ed.). Chicester: John Wiley and Sons Ltd. pp. 243–244.ISBN978-0-470-65624-2.
- ^Wotherspoon, AC; Doglioni, C; Diss, TC; Pan, L; Moschini, A; de Boni, M; Isaacson, PG (4 September 1993). "Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori".Lancet.342(8871): 575–7.doi:10.1016/0140-6736(93)91409-f.PMID8102719.S2CID9531600.
- ^Parsonnet J,Hansen S, Rodriguez L, Gelb A, Warnke R, Jellum E, Orentreich N, Vogelman J, Friedman G (1994)."Helicobacter pyloriinfection and gastric lymphoma ".N Engl J Med.330(18): 1267–71.doi:10.1056/NEJM199405053301803.PMID8145781.
- ^Hatakeyama, M.; Higashi, H. (2005)."Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis".Cancer Science.96(12): 835–843.doi:10.1111/j.1349-7006.2005.00130.x.PMC11159386.PMID16367902.
- ^ab"MALT Lymphoma".The Lecturio Medical Concept Library.Retrieved10 August2021.
- ^Bayerdörffer E, Oertel H, Lehn N, Kasper G, Mannes GA, Sauerbruch T, Stolte M (August 1989)."Topographic association between active gastritis and Campylobacter pylori colonisation".J. Clin. Pathol.42(8): 834–9.doi:10.1136/jcp.42.8.834.PMC1142061.PMID2768523.
- ^Park, Jeong Bae (2014)."infection in gastric mucosa-associated lymphoid tissue lymphoma".World Journal of Gastroenterology.20(11): 2751–9.doi:10.3748/wjg.v20.i11.2751.PMC3961970.PMID24659867.
- ^Fischbach, W; Goebeler, M E; Ruskone-Fourmestraux, A; Wundisch, T; Neubauer, A; Raderer, M; Savio, A (1 December 2007)."Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series".Gut.56(12): 1685–1687.doi:10.1136/gut.2006.096420.PMC2095715.PMID17639089.
- ^Sarah, Silverman."MALT lymphoma Diagnosis, Staging, Treatment".pylori.org.UEG. Archived fromthe originalon 2015-01-05.Retrieved2015-01-05.
- ^Noels H, van Loo G, Hagens S, et al. (April 2007)."A Novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-kappaB activation by API2middle dotMALT1 fusions".J. Biol. Chem.282(14): 10180–9.doi:10.1074/jbc.M611038200.PMID17287209.
- ^Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, Hamoudi RA, Diss TC, Dogan A, Megraud F, Rambaud JC, Du MQ, Isaacson PG (January 2001). "Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy".Lancet.357(9249): 39–40.doi:10.1016/S0140-6736(00)03571-6.PMID11197361.S2CID22237525.
- ^Tomita N, Kodaira T, Tachibana H, Nakamura T, Mizoguchi N, Takada A (February 2009). "Favorable outcomes of radiotherapy for early-stage mucosa-associated lymphoid tissue lymphoma".Radiother Oncol.90(2): 231–5.doi:10.1016/j.radonc.2008.12.004.PMID19135751.
- ^Schechter NR, Portlock CS, Yahalom J (May 1998). "Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone".J. Clin. Oncol.16(5): 1916–21.doi:10.1200/JCO.1998.16.5.1916.PMID9586910.
- ^Avilés A, Nambo MJ, Neri N, Talavera A, Cleto S (2005). "Mucosa-associated lymphoid tissue (MALT) lymphoma of the stomach: results of a controlled clinical trial".Med. Oncol.22(1): 57–62.doi:10.1385/MO:22:1:057.PMID15750197.S2CID29541664.
- ^Conconi A, Martinelli G, Thiéblemont C, Ferreri AJ, Devizzi L, Peccatori F, et al. (October 2003)."Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type".Blood.102(8): 2741–5.doi:10.1182/blood-2002-11-3496.PMID12842999.
- ^Martinelli G, Laszlo D, Ferreri AJ, Pruneri G, Ponzoni M, Conconi A, et al. (March 2005). "Clinical activity of rituximab in gastric marginal zone non-Hodgkin's lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy".J. Clin. Oncol.23(9): 1979–83.doi:10.1200/JCO.2005.08.128.PMID15668468.S2CID2572398.
- ^Ferreri, Andrés J. M.; Sassone, Marianna; Miserocchi, Elisabetta; Govi, Silvia; Cecchetti, Caterina; Corti, Matteo E.; Mappa, Silvia; Arcaini, Luca; Zaja, Francesco; Todeschini, Giuseppe; Mannina, Donato; Calimeri, Teresa; Perrone, Salvatore; Ponzoni, Maurilio; Modorati, Giulio (2020-03-24)."Treatment of MALT lymphoma of the conjunctiva with intralesional rituximab supplemented with autologous serum".Blood Advances.4(6): 1013–1019.doi:10.1182/bloodadvances.2020001459.ISSN2473-9537.PMC7094013.PMID32182364.
- ^Lévy M, Copie-Bergman C, Gameiro C, Chaumette MT, Delfau-Larue MH, Haioun C, et al. (August 2005)."Prognostic value of translocation t(11;18) in tumoral response of low-grade gastric lymphoma of mucosa-associated lymphoid tissue type to oral chemotherapy".J. Clin. Oncol.23(22): 5061–6.doi:10.1200/JCO.2005.05.660.PMID16051953.
- ^Zinzani PL, Stefoni V, Musuraca G, Tani M, Alinari L, Gabriele A, et al. (May 2004)."Fludarabine-containing chemotherapy as frontline treatment of nongastrointestinal mucosa-associated lymphoid tissue lymphoma".Cancer.100(10): 2190–4.doi:10.1002/cncr.20237.PMID15139063.S2CID25251522.
- ^Jäger G, Neumeister P, Quehenberger F, Wöhrer S, Linkesch W, Raderer M (November 2006)."Prolonged clinical remission in patients with extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type treated with cladribine: 6 year follow-up of a phase II trial".Ann. Oncol.17(11): 1722–3.doi:10.1093/annonc/mdl126.PMID16766585.
- ^Zucca E, Conconi A, Laszlo D, López-Guillermo A, Bouabdallah R, Coiffier B, et al. (February 2013). "Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study".J. Clin. Oncol.31(5): 565–72.doi:10.1200/JCO.2011.40.6272.hdl:11573/512880.PMID23295789.
- ^Ye H, Gong L, Liu H, Hamoudi RA, Shirali S, Ho L, et al. (February 2005). "MALT lymphoma with t(14;18)(q32;q21)/IGH-MALT1 is characterized by strong cytoplasmic MALT1 and BCL10 expression".J. Pathol.205(3): 293–301.doi:10.1002/path.1715.PMID15682443.S2CID41038826.
- ^Turgeon, Mary Louise (2005).Clinical hematology: theory and procedures.Hagerstown, MD: Lippincott Williams & Wilkins. p. 283.ISBN0-7817-5007-5.
Frequency of lymphoid neoplasms. (Source: Modified from WHO Blue Book on Tumour of Hematopoietic and Lymphoid Tissues. 2001, p. 2001.)
