MHC class I
| MHC class I | |
|---|---|
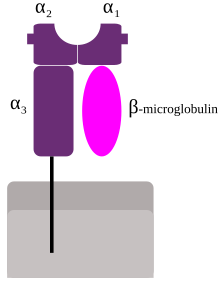 Schematic representation of MHC class I | |
| Identifiers | |
| Symbol | MHC class I |
| Membranome | 63 |
MHC class I moleculesare one of two primary classes ofmajor histocompatibility complex(MHC) molecules (the other beingMHC class II) and are found on thecell surfaceof allnucleatedcells in the bodies ofvertebrates.[1][2]They also occur onplatelets,but not onred blood cells.Their function is to display peptide fragments of proteins from within the cell tocytotoxic T cells;this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules presentpeptidesderived fromcytosolicproteins, the pathway of MHC class I presentation is often calledcytosolicorendogenous pathway.[3]
In humans, theHLAscorresponding to MHC class I areHLA-A,HLA-B,andHLA-C.
Function
[edit]Class I MHC molecules bindpeptidesgenerated mainly from degradation of cytosolic proteins by theproteasome.The MHC I:peptide complex is then inserted via endoplasmic reticulum into the external plasma membrane of the cell. The epitope peptide is bound on extracellular parts of the class I MHC molecule. Thus, the function of the class I MHC is to display intracellular proteins tocytotoxic T cells(CTLs). However, class I MHC can also present peptides generated from exogenous proteins, in a process known ascross-presentation.
A normal cell will display peptides from normal cellular protein turnover on its class I MHC, and CTLs will not be activated in response to them due to central and peripheral tolerance mechanisms. When a cell expresses foreign proteins, such as after viral infection, a fraction of the class I MHC will display these peptides on the cell surface. Consequently, CTLs specific for the MHC:peptide complex will recognize and kill presenting cells.
Alternatively, class I MHC itself can serve as an inhibitory ligand fornatural killer cells(NKs). Reduction in the normal levels of surface class I MHC, a mechanism employed by some viruses[4]and certain tumors to evade CTL responses, activates NK cell killing.
PirB and visual plasticity
[edit]Paired-immunoglobulin-like receptor B (PirB), an MHCI-binding receptor, is involved in the regulation of visualplasticity.[5]PirB is expressed in thecentral nervous systemand diminishesocular dominanceplasticityin the developmentalcritical periodand adulthood.[5]When the function of PirB was abolished in mutant mice,ocular dominanceplasticitybecame more pronounced at all ages.[5]PirB loss of function mutant mice also exhibited enhancedplasticityafter monocular deprivation during thecritical period.[5]These results suggest PirB may be involved in modulation ofsynaptic plasticityin thevisual cortex.
Structure
[edit]MHC class I molecules are heterodimers that consist of two polypeptide chains, α andβ2-microglobulin(B2M). The two chains are linked noncovalently via interaction of B2M and the α3domain. Only the α chain is polymorphic and encoded by aHLA gene,while the B2M subunit is not polymorphic and encoded by theBeta-2 microglobulingene. The α3domain is plasma membrane-spanning and interacts with theCD8co-receptor ofT-cells.The α3-CD8 interaction holds the MHC I molecule in place while theT cell receptor(TCR) on the surface of the cytotoxic T cell binds its α1-α2heterodimer ligand, and checks the coupled peptide for antigenicity. The α1and α2domains fold to make up a groove for peptides to bind. MHC class I molecules bind peptides that are predominantly 8-10 amino acid in length (Parham 87), but the binding of longer peptides have also been reported.[6]
While a high-affinity peptide and the B2M subunit are normally required to maintain a stableternary complexbetween the peptide, MHC I, and B2M, under subphysiological temperatures, stable, peptide-deficient MHC I/B2Mheterodimershave been observed.[7][8]Synthetic stable, peptide-receptive MHC I molecules have been generated using adisulfide bondbetween the MHC I and B2M, named "open MHC-I".[9]
Synthesis
[edit]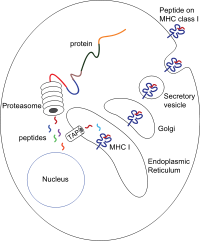
The peptides are generated mainly in thecytosolby theproteasome.The proteasome is a macromolecule that consists of 28 subunits, of which half affectproteolyticactivity. The proteasome degrades intracellular proteins into small peptides that are then released into the cytosol. Proteasomes can also ligate distinct peptide fragments (termed spliced peptides), producing sequences that are noncontiguous and therefore not linearly templated in the genome. The origin of spliced peptide segments can be from the same protein (cis-splicing) or different proteins (trans-splicing).[10][11]The peptides have to be translocated from the cytosol into theendoplasmic reticulum(ER) to meet the MHC class I molecule, whose peptide-binding site is in thelumenof the ER. They have membrane proximalIg fold
Translocation and peptide loading
[edit]The peptide translocation from the cytosol into the lumen of the ER is accomplished by thetransporter associated with antigen processing(TAP). TAP is a member of theABC transporterfamily and is a heterodimeric multimembrane-spanning polypeptide consisting ofTAP1andTAP2.The two subunits form a peptide binding site and two ATP binding sites that face the cytosol. TAP binds peptides on the cytoplasmic side and translocates them underATPconsumption into the lumen of the ER. The MHC class I molecule is then, in turn, loaded with peptides in the lumen of the ER.
The peptide-loading process involves several other molecules that form a large multimeric complex called thePeptide loading complex[12]consisting of TAP,tapasin,calreticulin,calnexin,andErp57(PDIA3). Calnexin acts to stabilize the class I MHC α chains prior to β2m binding. Following complete assembly of the MHC molecule, calnexin dissociates. The MHC molecule lacking a bound peptide is inherently unstable and requires the binding of the chaperones calreticulin and Erp57. Additionally, tapasin binds to the MHC molecule and serves to link it to the TAP proteins and facilitates the selection of peptide in an iterative process called peptide editing,[13][14][15]thus facilitating enhanced peptide loading and colocalization.
Once the peptide is loaded onto the MHC class I molecule, the complex dissociates and it leaves the ER through thesecretory pathwayto reach the cell surface. The transport of the MHC class I molecules through the secretory pathway involves severalposttranslational modificationsof the MHC molecule. Some of the posttranslational modifications occur in the ER and involve change to theN-glycanregions of the protein, followed by extensive changes to the N-glycans in theGolgi apparatus.The N-glycans mature fully before they reach the cell surface.
Peptide removal
[edit]Peptides that fail to bind MHC class I molecules in the lumen of the endoplasmic reticulum (ER) are removed from the ER via thesec61channel into the cytosol,[16][17]where they might undergo further trimming in size, and might be translocated by TAP back into ER for binding to a MHC class I molecule.
For example, an interaction of sec61 with bovinealbuminhas been observed.[18]
Effect of viruses
[edit]MHC class I molecules are loaded with peptides generated from the degradation ofubiquitinatedcytosolic proteins inproteasomes.As viruses induce cellular expression of viral proteins, some of these products are tagged for degradation, with the resulting peptide fragments entering the endoplasmic reticulum and binding to MHC I molecules. It is in this way, the MHC class I-dependent pathway of antigen presentation, that the virus infected cells signal T-cells that abnormal proteins are being produced as a result of infection.
The fate of the virus-infected cell is almost always induction ofapoptosisthroughcell-mediated immunity,reducing the risk of infecting neighboring cells. As an evolutionary response to this method of immune surveillance, many viruses are able to down-regulate or otherwise prevent the presentation of MHC class I molecules on the cell surface. In contrast to cytotoxic T lymphocytes,natural killer(NK) cells are normally inactivated upon recognizing MHC I molecules on the surface of cells. Therefore, in the absence of MHC I molecules, NK cells are activated and recognize the cell as aberrant, suggesting that it may be infected by viruses attempting to evade immune destruction. Several human cancers also show down-regulation of MHC I, giving transformed cells the same survival advantage of being able to avoid normal immune surveillance designed to destroy any infected or transformed cells.[19]
Genes and isotypes
[edit]- Very polymorphic
- Less polymorphic
Evolutionary history
[edit]The MHC class I genes originated in the mostrecent common ancestorof alljawed vertebrates,and have been found in all living jawed vertebrates that have been studied thus far.[2]Since their emergence in jawed vertebrates, this gene family has been subjected to many divergent evolutionary paths asspeciationevents have taken place. There are, however, documented cases of trans-speciespolymorphismsin MHC class I genes, where a particularallelein an evolutionary related MHC class I gene remains in two species, likely due to strong pathogen-mediatedbalancing selectionbypathogensthat can infect both species.[20]Birth-and-deathevolution is one of the mechanistic explanations for the size of the MHC class I gene family.
Birth-and-death of MHC class I genes
[edit]Birth-and-death evolution asserts thatgene duplicationevents cause the genome to contain multiple copies of a gene which can then undergo separate evolutionary processes. Sometimes these processes result inpseudogenization(death) of one copy of the gene, though sometimes this process results in two new genes with divergent function.[21]It is likely that human MHC class Ib loci (HLA-E, -F, and -G) as well as MHC class I pseudogenes arose from MHC class Ia loci (HLA-A, -B, and -C) in this birth-and-death process.[22]
References
[edit]- ^Hewitt EW (October 2003)."The MHC class I antigen presentation pathway: strategies for viral immune evasion".Immunology.110(2): 163–9.doi:10.1046/j.1365-2567.2003.01738.x.PMC1783040.PMID14511229.
- ^abKulski JK, Shiina T, Anzai T, Kohara S, Inoko H (December 2002). "Comparative genomic analysis of the MHC: the evolution of class I duplication blocks, diversity and complexity from shark to man".Immunological Reviews.190:95–122.doi:10.1034/j.1600-065x.2002.19008.x.PMID12493009.S2CID41765680.
- ^http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/H/HLA.html#Class_I_Histocompatibility_MoleculesArchived2016-02-04 at theWayback MachineKimball's Biology Pages, Histocompatibility Molecules
- ^Hansen TH, Bouvier M (July 2009). "MHC class I antigen presentation: learning from viral evasion strategies".Nature Reviews. Immunology.9(7): 503–13.doi:10.1038/nri2575.PMID19498380.S2CID9278263.
- ^abcdSyken J, Grandpre T, Kanold PO, Shatz CJ (September 2006). "PirB restricts ocular-dominance plasticity in visual cortex".Science.313(5794): 1795–800.Bibcode:2006Sci...313.1795S.doi:10.1126/science.1128232.PMID16917027.S2CID1860730.
- ^Burrows SR, Rossjohn J, McCluskey J (January 2006). "Have we cut ourselves too short in mapping CTL epitopes?".Trends in Immunology.27(1): 11–6.doi:10.1016/j.it.2005.11.001.PMID16297661.
- ^Ljunggren HG, Stam NJ, Öhlén C, Neefjes JJ, Höglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Kärre K, Ploegh HL (1990-08-02)."Empty MHC class I molecules come out in the cold".Nature.346(6283): 476–480.Bibcode:1990Natur.346..476L.doi:10.1038/346476a0.ISSN0028-0836.PMID2198471.
- ^Schumacher TN, Heemels MT, Neefjes JJ, Kast W, Melief CJ, Ploegh HL (August 1990)."Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro".Cell.62(3): 563–567.doi:10.1016/0092-8674(90)90020-F.PMID2199065.
- ^Sun Y, Young MC, Woodward CH, Danon JN, Truong HV, Gupta S, Winters TJ, Font-Burgada J, Burslem GM, Sgourakis NG (2023-06-20)."Universal open MHC-I molecules for rapid peptide loading and enhanced complex stability across HLA allotypes".Proceedings of the National Academy of Sciences.120(25): e2304055120.Bibcode:2023PNAS..12004055S.doi:10.1073/pnas.2304055120.ISSN0027-8424.PMC10288639.PMID37310998.
- ^Faridi P, Li C, Ramarathinam SH, Vivian JP, Illing PT, Mifsud NA, Ayala R, Song J, Gearing LJ, Hertzog PJ, Ternette N, Rossjohn J, Croft NP, Purcell AW (12 October 2018)."A subset of HLA-I peptides are not genomically templated: Evidence for cis- and trans-spliced peptide ligands"(PDF).Science Immunology.3(28): eaar3947.doi:10.1126/sciimmunol.aar3947.PMID30315122.
- ^Liepe J, Marino F, Sidney J, Jeko A, Bunting DE, Sette A, Kloetzel PM, Stumpf MP, Heck AJ, Mishto M (21 October 2016)."A large fraction of HLA class I ligands are proteasome-generated spliced peptides"(PDF).Science.354(6310): 354–358.Bibcode:2016Sci...354..354L.doi:10.1126/science.aaf4384.hdl:10044/1/42330.PMID27846572.S2CID41095551.
- ^Blees A, Januliene D, Hofmann T, Koller N, Schmidt C, Trowitzsch S, Moeller A, Tampé R (November 2017). "Structure of the human MHC-I peptide-loading complex".Nature.551(7681): 525–528.Bibcode:2017Natur.551..525B.doi:10.1038/nature24627.PMID29107940.S2CID4447406.
- ^Howarth M, Williams A, Tolstrup AB, Elliott T (August 2004)."Tapasin enhances MHC class I peptide presentation according to peptide half-life".Proceedings of the National Academy of Sciences of the United States of America.101(32): 11737–42.Bibcode:2004PNAS..10111737H.doi:10.1073/pnas.0306294101.PMC511045.PMID15286279.
- ^Wearsch PA, Cresswell P (August 2007). "Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer".Nature Immunology.8(8): 873–81.doi:10.1038/ni1485.PMID17603487.S2CID29762957.
- ^Thirdborough SM, Roddick JS, Radcliffe JN, Howarth M, Stevenson FK, Elliott T (February 2008)."Tapasin shapes immunodominance hierarchies according to the kinetic stability of peptide-MHC class I complexes".European Journal of Immunology.38(2): 364–9.doi:10.1002/eji.200737832.PMID18196518.S2CID28659293.
- ^Koopmann JO, Albring J, Hüter E, Bulbuc N, Spee P, Neefjes J, Hämmerling GJ, Momburg F, et al. (July 2000)."Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel".Immunity.13(1): 117–27.doi:10.1016/S1074-7613(00)00013-3.PMID10933400.
- ^Albring J, Koopmann JO, Hämmerling GJ, Momburg F (January 2004). "Retrotranslocation of MHC class I heavy chain from the endoplasmic reticulum to the cytosol is dependent on ATP supply to the ER lumen".Molecular Immunology.40(10): 733–41.doi:10.1016/j.molimm.2003.08.008.PMID14644099.
- ^Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I (January 2005)."Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells".International Immunology.17(1): 45–53.doi:10.1093/intimm/dxh184.PMID15546887.
- ^Wang Z, Zhang L, Qiao A, Watson K, Zhang J, Fan GH (February 2008)."Activation of CXCR4 triggers ubiquitination and down-regulation of major histocompatibility complex class I (MHC-I) on epithelioid carcinoma HeLa cells".The Journal of Biological Chemistry.283(7): 3951–9.doi:10.1074/jbc.m706848200.PMID18083706.
- ^Azevedo L, Serrano C, Amorim A, Cooper DN (September 2015)."Trans-species polymorphism in humans and the great apes is generally maintained by balancing selection that modulates the host immune response".Human Genomics.9(1): 21.doi:10.1186/s40246-015-0043-1.PMC4559023.PMID26337052.
- ^Nei M, Rooney AP (2005-11-14)."Concerted and birth-and-death evolution of multigene families".Annual Review of Genetics.39(1): 121–52.doi:10.1146/annurev.genet.39.073003.112240.PMC1464479.PMID16285855.
- ^Hughes AL (March 1995)."Origin and evolution of HLA class I pseudogenes".Molecular Biology and Evolution.12(2): 247–58.doi:10.1093/oxfordjournals.molbev.a040201.PMID7700152.
External links
[edit]- Histocompatibility+Antigens+Class+Iat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- MHC+Class+I+Genesat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
