MK-2206
| |
| Names | |
|---|---|
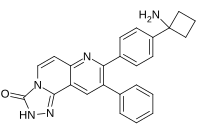
| Preferred IUPAC name
8-[4-(1-Aminocyclobutyl)phenyl]-9-phenyl[1,2,4]triazolo[3,4-f] [1,6]naphthyridin-3(2H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.207.435 |
| EC Number |
|
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C25H21N5O | |
| Molar mass | 407.477g·mol−1 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
MK-2206is a drug candidate being investigated to help treat cancer. Its chemical formula is C25H21N5O.[1]It acts as anallostericAKT inhibitor.[2]
It is a highly selective inhibitor of pan-Akt, namely, of all three Akt isoformsAkt1,Akt2,andAkt3.[1]
It is intended to be used with other cancer therapies that advanced tumours may become resistant to.[3]
Clinical trials
[edit]2011: A phase 1 clinical trial of MK-2206 alone has reported it was well tolerated.[4]
2014: A phase 1 clinical trial of MK-2206 with a variety of other agents in 72 patients with advanced cancer reported acceptable side-effects.[3]
2016: MK-2206 is one of the treatments in theI-SPY2Adaptive clinical trialforbreast cancerthat had been selected for later stage trials.[5]
As of August 2017[update]31 phase II clinical trials are registered, many completed.[6]e.g. in colorectal cancer, breast cancer, and many others.
MK-2206 and COVID-19
[edit]Data shown in a studypreprintsuggest thatSARS-CoV-2infection decreases cellularautophagyand that MK-2206, which induces autophagy, reduced virus replication by up to 88%in vitro.The study's authors propose that MK-2206 should be tested inclinical trialsas a potential treatment for COVID-19.[7]
References
[edit]- ^abMK-2206 dihydrochloride (CAS 1032350-13-2)inc structure diagram
- ^MK-2206, an Allosteric Akt Inhibitor, Enhances Antitumor Efficacy by Standard Chemotherapeutic Agents or Molecular Targeted Drugs In vitro and In vivo. Hirai et al. 2010
- ^abA trial of MK-2206 with chemotherapy or erlotinib for advanced cancer
- ^Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. (December 2011). "First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors".Journal of Clinical Oncology.29(35): 4688–4695.doi:10.1200/JCO.2011.35.5263.PMID22025163.
- ^Novel Agents are Targeting Drivers of TNBC - Several drug candidates in I-SPY2 have 'graduated' to later-phase studies. June 2016
- ^MK-2206 phase=2 trials
- ^Gassen NC, Papies J, Bajaj T, Emanuel J, Dethloff F, Chua RL, et al. (June 2021)."SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals".Nature Communications.12(1): 3818.doi:10.1038/s41467-021-24007-w.PMC8217552.PMID34155207.
