Messenger RNA
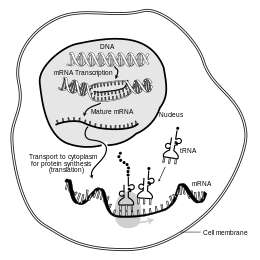
Inmolecular biology,messenger ribonucleic acid(mRNA) is a single-strandedmoleculeofRNAthat corresponds to thegenetic sequenceof agene,and is read by aribosomein the process ofsynthesizingaprotein.
mRNA is created during the process oftranscription,where anenzyme(RNA polymerase) converts the gene intoprimary transcriptmRNA (also known aspre-mRNA). This pre-mRNA usually still containsintrons,regions that will not go on to code for the finalamino acid sequence.These are removed in the process ofRNA splicing,leaving onlyexons,regions that will encode the protein. This exon sequence constitutesmature mRNA.Mature mRNA is then read by the ribosome, and the ribosome creates the protein utilizingamino acidscarried bytransfer RNA(tRNA). This process is known astranslation.All of these processes form part of thecentral dogma of molecular biology,which describes the flow of genetic information in a biological system.
As inDNA,genetic information in mRNA is contained in the sequence ofnucleotides,which are arranged intocodonsconsisting of threeribonucleotideseach. Each codon codes for a specificamino acid,except thestop codons,which terminate protein synthesis. The translation of codons into amino acids requires two other types of RNA: transfer RNA, which recognizes the codon and provides the corresponding amino acid, andribosomal RNA(rRNA), the central component of the ribosome's protein-manufacturing machinery.
The concept of mRNA was developed bySydney BrennerandFrancis Crickin 1960 during a conversation withFrançois Jacob.In 1961, mRNA was identified and described independently by one team consisting of Brenner, Jacob, andMatthew Meselson,and another team led byJames Watson.While analyzing the data in preparation for publication, Jacob andJacques Monodcoined the name "messenger RNA".
Synthesis
[edit]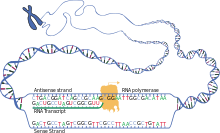
The brief existence of an mRNA molecule begins with transcription, and ultimately ends in degradation. During its life, an mRNA molecule may also be processed, edited, and transported prior to translation. Eukaryotic mRNA molecules often require extensive processing and transport, whileprokaryoticmRNA molecules do not. A molecule ofeukaryoticmRNA and the proteins surrounding it are together called amessenger RNP.[citation needed]
Transcription
[edit]Transcription is when RNA is copied from DNA. During transcription,RNA polymerasemakes a copy of a gene from the DNA to mRNA as needed. This process differs slightly in eukaryotes and prokaryotes. One notable difference is that prokaryotic RNA polymerase associates with DNA-processing enzymes during transcription so that processing can proceed during transcription. Therefore, this causes the new mRNA strand to become double stranded by producing a complementary strand known as the tRNA strand, which when combined are unable to form structures from base-pairing. Moreover, the template for mRNA is the complementary strand of tRNA, which is identical in sequence to the anticodon sequence that the DNA binds to. The short-lived, unprocessed or partially processed product is termedprecursor mRNA,orpre-mRNA;once completely processed, it is termedmature mRNA.[citation needed]
Uracil substitution for thymine
[edit]mRNA uses uracil (U) instead of thymine (T) in DNA. uracil (U) is the complimentary base to adenine (A) during transcription instead of thymine (T). So when using a template strand of DNA to build RNA, thymine is replaced with uracil. This substitution allows the mRNA to carry the appropriate genetic information from DNA to the ribosome for translation. Regarding the natural history, uracil came first than thymine; evidence suggests that RNA came before DNA in evolution.[1]The RNA World hypothesis proposes that life began with RNA molecules, before the emergence of DNA genomes and coded proteins. In DNA, the evolutionary substitution of thymine for uracil may have increased DNA stability and improved the efficiency of DNA replication.[2][3]
Eukaryotic pre-mRNA processing
[edit]
Processing of mRNA differs greatly amongeukaryotes,bacteria,andarchaea.Non-eukaryotic mRNA is, in essence, mature upon transcription and requires no processing, except in rare cases.[4]Eukaryotic pre-mRNA, however, requires several processing steps before its transport to the cytoplasm and its translation by the ribosome.
Splicing
[edit]The extensive processing of eukaryotic pre-mRNA that leads to the mature mRNA is theRNA splicing,a mechanism by whichintronsoroutrons(non-coding regions) are removed andexons(coding regions) are joined.[citation needed]
5' cap addition
[edit]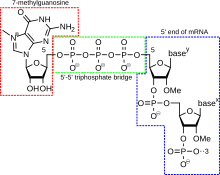
A5' cap(also termed an RNA cap, an RNA7-methylguanosinecap, or an RNA m7G cap) is a modified guanine nucleotide that has been added to the "front" or5' endof a eukaryotic messenger RNA shortly after the start of transcription. The 5' cap consists of a terminal 7-methylguanosine residue that is linked through a 5'-5'-triphosphate bond to the first transcribed nucleotide. Its presence is critical for recognition by theribosomeand protection fromRNases.[citation needed]
Cap addition is coupled to transcription, and occurs co-transcriptionally, such that each influences the other. Shortly after the start of transcription, the 5' end of the mRNA being synthesized is bound by acap-synthesizing complexassociated withRNA polymerase.Thisenzymaticcomplexcatalyzesthe chemical reactions that are required for mRNA capping. Synthesis proceeds as a multi-stepbiochemicalreaction.[citation needed]
Editing
[edit]In some instances, an mRNA will beedited,changing the nucleotide composition of that mRNA. An example in humans is theapolipoprotein BmRNA, which is edited in some tissues, but not others. The editing creates an early stop codon, which, upon translation, produces a shorter protein.
Polyadenylation
[edit]
Polyadenylation is the covalent linkage of a polyadenylyl moiety to a messenger RNA molecule. In eukaryotic organisms most messenger RNA (mRNA) molecules are polyadenylated at the 3' end, but recent studies have shown that short stretches of uridine (oligouridylation) are also common.[5]Thepoly(A) tailand the protein bound to it aid in protecting mRNA from degradation by exonucleases. Polyadenylation is also important for transcription termination, export of the mRNA from the nucleus, and translation. mRNA can also be polyadenylated in prokaryotic organisms, where poly(A) tails act to facilitate, rather than impede, exonucleolytic degradation.[citation needed]
Polyadenylation occurs during and/or immediately after transcription of DNA into RNA. After transcription has been terminated, the mRNA chain is cleaved through the action of an endonuclease complex associated with RNA polymerase. After the mRNA has been cleaved, around 250 adenosine residues are added to the free 3' end at the cleavage site. This reaction is catalyzed bypolyadenylate polymerase.Just as inalternative splicing,there can be more than one polyadenylation variant of an mRNA.
Polyadenylation site mutations also occur. The primary RNA transcript of a gene is cleaved at the poly-A addition site, and 100–200 A's are added to the 3' end of the RNA. If this site is altered, an abnormally long and unstable mRNA construct will be formed.
Transport
[edit]Another difference between eukaryotes and prokaryotes is mRNA transport. Because eukaryotic transcription and translation is compartmentally separated, eukaryotic mRNAs must be exported from thenucleusto thecytoplasm—a process that may be regulated by different signaling pathways.[6]Mature mRNAs are recognized by their processed modifications and then exported through thenuclear poreby binding to the cap-binding proteins CBP20 and CBP80,[7]as well as the transcription/export complex (TREX).[8][9]Multiple mRNA export pathways have been identified in eukaryotes.[10]
In spatially complex cells, some mRNAs are transported to particular subcellular destinations. In matureneurons,certain mRNA are transported from thesomatodendrites.One site of mRNA translation is at polyribosomes selectively localized beneath synapses.[11]The mRNA forArc/Arg3.1is induced by synaptic activity and localizes selectively near activesynapsesbased on signals generated byNMDA receptors.[12]Other mRNAs also move into dendrites in response to external stimuli, such asβ-actinmRNA.[13]For export from the nucleus, actin mRNA associates withZBP1[14]and later with40S subunit.The complex is bound by amotor proteinand is transported to the target location (neurite extension) along thecytoskeleton.Eventually ZBP1 isphosphorylatedbySrcin order for translation to be initiated.[15]In developing neurons, mRNAs are also transported into growingaxonsand especially growth cones. Many mRNAs are marked with so-called "zip codes", which target their transport to a specific location.[16][17]mRNAs can also transfer between mammalian cells through structures calledtunneling nanotubes.[18][19]
Translation
[edit]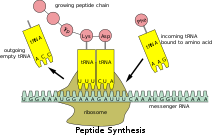
Because prokaryotic mRNA does not need to be processed or transported, translation by theribosomecan begin immediately after the end of transcription. Therefore, it can be said that prokaryotic translation iscoupledto transcription and occursco-transcriptionally.[citation needed]
Eukaryotic mRNA that has been processed and transported to the cytoplasm (i.e., mature mRNA) can then be translated by the ribosome. Translation may occur at ribosomes free-floating in the cytoplasm, or directed to theendoplasmic reticulumby thesignal recognition particle.Therefore, unlike in prokaryotes, eukaryotic translationis notdirectly coupled to transcription. It is even possible in some contexts that reduced mRNA levels are accompanied by increased protein levels, as has been observed for mRNA/protein levels ofEEF1A1inbreast cancer.[20][non-primary source needed]
Structure
[edit]
Coding regions
[edit]Coding regions are composed ofcodons,which are decoded and translated into proteins by the ribosome; in eukaryotes usually into one and in prokaryotes usually into several. Coding regions begin with thestart codonand end with astop codon.In general, the start codon is an AUG triplet and the stop codon is UAG ( "amber" ), UAA ( "ochre" ), or UGA ( "opal" ). The coding regions tend to be stabilised by internal base pairs; this impedes degradation.[21][22]In addition to being protein-coding, portions of coding regions may serve as regulatory sequences in thepre-mRNAasexonic splicing enhancersorexonic splicing silencers.
Untranslated regions
[edit]
Untranslated regions (UTRs) are sections of the mRNA before the start codon and after the stop codon that are not translated, termed thefive prime untranslated region(5' UTR) andthree prime untranslated region(3' UTR), respectively. These regions are transcribed with the coding region and thus areexonicas they are present in the mature mRNA. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, andtranslational efficiency.The ability of a UTR to perform these functions depends on the sequence of the UTR and can differ between mRNAs. Genetic variants in 3' UTR have also been implicated in disease susceptibility because of the change in RNA structure and protein translation.[23]
The stability of mRNAs may be controlled by the 5' UTR and/or 3' UTR due to varying affinity for RNA degrading enzymes calledribonucleasesand for ancillary proteins that can promote or inhibit RNA degradation. (See also,C-rich stability element.)
Translational efficiency, including sometimes the complete inhibition of translation, can be controlled by UTRs. Proteins that bind to either the 3' or 5' UTR may affect translation by influencing the ribosome's ability to bind to the mRNA.MicroRNAsbound to the3' UTRalso may affect translational efficiency or mRNA stability.
Cytoplasmic localization of mRNA is thought to be a function of the 3' UTR. Proteins that are needed in a particular region of the cell can also be translated there; in such a case, the 3' UTR may contain sequences that allow the transcript to be localized to this region for translation.
Some of the elements contained in untranslated regions form a characteristicsecondary structurewhen transcribed into RNA. These structural mRNA elements are involved in regulating the mRNA. Some, such as theSECIS element,are targets for proteins to bind. One class of mRNA element, theriboswitches,directly bind small molecules, changing their fold to modify levels of transcription or translation. In these cases, the mRNA regulates itself.
Poly(A) tail
[edit]The 3' poly(A) tail is a long sequence ofadeninenucleotides (often several hundred) added to the3' endof the pre-mRNA. This tail promotes export from the nucleus and translation, and protects the mRNA from degradation.
Monocistronic versus polycistronic mRNA
[edit]An mRNA molecule is said to be monocistronic when it contains the genetic information totranslateonly a singleproteinchain (polypeptide). This is the case for most of theeukaryoticmRNAs.[24][25]On the other hand, polycistronic mRNA carries severalopen reading frames(ORFs), each of which is translated into a polypeptide. These polypeptides usually have a related function (they often are the subunits composing a final complex protein) and their coding sequence is grouped and regulated together in a regulatory region, containing apromoterand anoperator.Most of the mRNA found inbacteriaandarchaeais polycistronic,[24]as is the human mitochondrial genome.[26]Dicistronic or bicistronic mRNA encodes only twoproteins.
mRNA circularization
[edit]
In eukaryotes mRNA molecules form circular structures due to an interaction between theeIF4Eandpoly(A)-binding protein,which both bind toeIF4G,forming an mRNA-protein-mRNA bridge.[27]Circularization is thought to promote cycling of ribosomes on the mRNA leading to time-efficient translation, and may also function to ensure only intact mRNA are translated (partially degraded mRNA characteristically have no m7G cap, or no poly-A tail).[28]
Other mechanisms for circularization exist, particularly in virus mRNA.PoliovirusmRNA uses a cloverleaf section towards its 5' end to bind PCBP2, which bindspoly(A)-binding protein,forming the familiar mRNA-protein-mRNA circle.Barley yellow dwarf virushas binding between mRNA segments on its 5' end and 3' end (called kissing stem loops), circularizing the mRNA without any proteins involved.
RNA virus genomes (the + strands of which are translated as mRNA) are also commonly circularized.[29]During genome replication the circularization acts to enhance genome replication speeds, cycling viral RNA-dependent RNA polymerase much the same as the ribosome is hypothesized to cycle.
Degradation
[edit]Different mRNAs within the same cell have distinct lifetimes (stabilities). In bacterial cells, individual mRNAs can survive from seconds to more than an hour. However, the lifetime averages between 1 and 3 minutes, making bacterial mRNA much less stable than eukaryotic mRNA.[30]In mammalian cells, mRNA lifetimes range from several minutes to days.[31]The greater the stability of an mRNA the more protein may be produced from that mRNA. The limited lifetime of mRNA enables a cell to alter protein synthesis rapidly in response to its changing needs. There are many mechanisms that lead to the destruction of an mRNA, some of which are described below.
Prokaryotic mRNA degradation
[edit]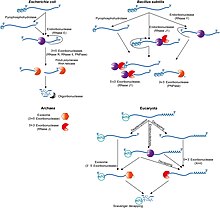
In general, in prokaryotes the lifetime of mRNA is much shorter than in eukaryotes. Prokaryotes degrade messages by using a combination of ribonucleases, includingendonucleases,3'exonucleases,and 5' exonucleases. In some instances,small RNA molecules(sRNA) tens to hundreds of nucleotides long can stimulate the degradation of specific mRNAs by base-pairing with complementary sequences and facilitating ribonuclease cleavage byRNase III.It was recently shown that bacteria also have a sort of5' capconsisting of a triphosphate on the5' end.[32]Removal of two of the phosphates leaves a 5' monophosphate, causing the message to be destroyed by the exonuclease RNase J, which degrades 5' to 3'.
Eukaryotic mRNA turnover
[edit]Inside eukaryotic cells, there is a balance between the processes oftranslationand mRNA decay. Messages that are being actively translated are bound byribosomes,theeukaryotic initiation factorseIF-4EandeIF-4G,andpoly(A)-binding protein.eIF-4E and eIF-4G block the decapping enzyme (DCP2), and poly(A)-binding protein blocks theexosome complex,protecting the ends of the message. The balance between translation and decay is reflected in the size and abundance of cytoplasmic structures known asP-bodies.[33]Thepoly(A) tailof the mRNA is shortened by specialized exonucleases that are targeted to specific messenger RNAs by a combination of cis-regulatory sequences on the RNA and trans-acting RNA-binding proteins. Poly(A) tail removal is thought to disrupt the circular structure of the message and destabilize thecap binding complex.The message is then subject to degradation by either theexosome complexor thedecapping complex.In this way, translationally inactive messages can be destroyed quickly, while active messages remain intact. The mechanism by which translation stops and the message is handed-off to decay complexes is not understood in detail.
AU-rich element decay
[edit]The presence ofAU-rich elementsin some mammalian mRNAs tends to destabilize those transcripts through the action of cellular proteins that bind these sequences and stimulatepoly(A)tail removal. Loss of the poly(A) tail is thought to promote mRNA degradation by facilitating attack by both theexosome complex[34]and thedecapping complex.[35]Rapid mRNA degradation viaAU-rich elementsis a critical mechanism for preventing the overproduction of potent cytokines such as tumor necrosis factor (TNF) and granulocyte-macrophage colony stimulating factor (GM-CSF).[36]AU-rich elements also regulate the biosynthesis of proto-oncogenic transcription factors likec-Junandc-Fos.[37]
Nonsense-mediated decay
[edit]Eukaryotic messages are subject to surveillance bynonsense-mediated decay(NMD), which checks for the presence of premature stop codons (nonsense codons) in the message. These can arise via incomplete splicing,V(D)J recombinationin theadaptive immune system,mutations in DNA, transcription errors,leaky scanningby the ribosome causing aframe shift,and other causes. Detection of a premature stop codon triggers mRNA degradation by 5' decapping, 3'poly(A)tail removal, orendonucleolytic cleavage.[38]
Small interfering RNA (siRNA)
[edit]Inmetazoans,small interfering RNAs(siRNAs) processed byDicerare incorporated into a complex known as theRNA-induced silencing complexor RISC. This complex contains anendonucleasethat cleaves perfectly complementary messages to which the siRNA binds. The resulting mRNA fragments are then destroyed byexonucleases.siRNA is commonly used in laboratories to block the function of genes in cell culture. It is thought to be part of the innate immune system as a defense against double-stranded RNA viruses.[39]
MicroRNA (miRNA)
[edit]MicroRNAs (miRNAs) are small RNAs that typically are partially complementary to sequences in metazoan messenger RNAs.[40][41]Binding of a miRNA to a message can repress translation of that message and accelerate poly(A) tail removal, thereby hastening mRNA degradation. The mechanism of action of miRNAs is the subject of active research.[42][43]
Other decay mechanisms
[edit]There are other ways by which messages can be degraded, includingnon-stop decayand silencing byPiwi-interacting RNA(piRNA), among others.
Applications
[edit]The administration of anucleoside-modified messenger RNAsequence can cause a cell to make a protein, which in turn could directly treat a disease or could function as avaccine;more indirectly the protein could drive an endogenousstem cellto differentiate in a desired way.[44][45]
The primary challenges of RNA therapy center on delivering the RNA to the appropriate cells.[46]Challenges include the fact that naked RNA sequences naturally degrade after preparation; they may trigger the body'simmune systemto attack them as an invader; and they areimpermeableto thecell membrane.[45]Once within the cell, they must then leave the cell's transport mechanism to take action within thecytoplasm,which houses the necessaryribosomes.[44]
Overcoming these challenges, mRNA as a therapeutic was first put forward in 1989 "after the development of a broadly applicable in vitro transfection technique."[47]In the 1990s, mRNA vaccines for personalized cancer have been developed, relying on non-nucleoside modified mRNA. mRNA based therapies continue to be investigated as a method of treatment or therapy for both cancer as well as auto-immune, metabolic, and respiratory inflammatory diseases. Gene editing therapies such asCRISPRmay also benefit from using mRNA to induce cells to make the desiredCasprotein.[48]
Since the 2010s, RNA vaccines and other RNA therapeutics have been considered to be "a new class of drugs".[49]The first mRNA-based vaccines received restricted authorization and were rolled out across the world during theCOVID-19 pandemicbyPfizer–BioNTech COVID-19 vaccineandModerna,for example.[50] The 2023Nobel Prize in Physiology or Medicinewas awarded toKatalin KarikóandDrew Weissmanfor the development of effective mRNA vaccines against COVID-19.[51][52][53]
History
[edit]Several molecular biology studies during the 1950s indicated that RNA played some kind of role in protein synthesis, but that role was not clearly understood. For instance, in one of the earliest reports,Jacques Monodand his team showed that RNA synthesis was necessary for protein synthesis, specifically during the production of the enzymeβ-galactosidasein the bacteriumE. coli.[54]Arthur Pardeealso found similar RNA accumulation in 1954.[55]In 1953,Alfred Hershey,June Dixon, andMartha Chasedescribed a certain cytosine-containing DNA (indicating it was RNA) that disappeared quickly after its synthesis inE. coli.[56]In hindsight, this may have been one of the first observations of the existence of mRNA but it was not recognized at the time as such.[57]
The idea of mRNA was first conceived bySydney BrennerandFrancis Crickon 15 April 1960 atKing's College, Cambridge,whileFrançois Jacobwas telling them about a recent experiment conducted byArthur Pardee,himself, and Monod (the so-called PaJaMo experiment, which did not prove mRNA existed but suggested the possibility of its existence). With Crick's encouragement, Brenner and Jacob immediately set out to test this new hypothesis, and they contactedMatthew Meselsonat theCalifornia Institute of Technologyfor assistance. During the summer of 1960, Brenner, Jacob, and Meselson conducted an experiment in Meselson's laboratory at Caltech which was the first to prove the existence of mRNA. That fall, Jacob and Monod coined the name "messenger RNA" and developed the first theoretical framework to explain its function.[57]
In February 1961,James Watsonrevealed that hisHarvard-based research group had been right behind them with a series of experiments whose results pointed in roughly the same direction. Brenner and the others agreed to Watson's request to delay publication of their research findings. As a result, the Brenner and Watson articles were published simultaneously in the same issue ofNaturein May 1961, while that same month, Jacob and Monod published their theoretical framework for mRNA in theJournal of Molecular Biology.[57]
See also
[edit]- Extension Poly(A) Test
- GeneCalling,an mRNA profiling technology
- Missense mRNA
- mRNA display
- mRNA surveillance
- Prokaryotic mRNA degradation
- Transcriptome,the sum of all RNA in a cell
References
[edit]- ^"The Information in DNA Is Decoded by Transcription | Learn Science at Scitable".www.nature.com.Retrieved2024-05-03.
- ^"RNA world (article) | Natural selection".Khan Academy.Retrieved2024-05-03.
- ^"The presence of thymine the place of uracil also confers additional stability to DNA. How?".Toppr Ask.Retrieved2024-05-04.
- ^Watson JD(February 22, 2013).Molecular Biology of the Gene, 7th edition.Pearson Higher Ed USA.ISBN9780321851499.
- ^Choi YS, Patena W, Leavitt AD, McManus MT (March 2012)."Widespread RNA 3'-end oligouridylation in mammals".RNA.18(3): 394–401.doi:10.1261/rna.029306.111.PMC3285928.PMID22291204.
- ^Quaresma AJ, Sievert R, Nickerson JA (April 2013)."Regulation of mRNA export by the PI3 kinase/AKT signal transduction pathway".Molecular Biology of the Cell.24(8): 1208–1221.doi:10.1091/mbc.E12-06-0450.PMC3623641.PMID23427269.
- ^Kierzkowski D, Kmieciak M, Piontek P, Wojtaszek P, Szweykowska-Kulinska Z, Jarmolowski A (September 2009)."The Arabidopsis CBP20 targets the cap-binding complex to the nucleus, and is stabilized by CBP80".The Plant Journal.59(5): 814–825.doi:10.1111/j.1365-313X.2009.03915.x.PMID19453442.
- ^Strässer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondón AG, Aguilera A, Struhl K, Reed R, Hurt E (May 2002). "TREX is a conserved complex coupling transcription with messenger RNA export".Nature.417(6886): 304–308.Bibcode:2002Natur.417..304S.doi:10.1038/nature746.PMID11979277.S2CID1112194.
- ^Katahira J, Yoneda Y (27 October 2014)."Roles of the TREX complex in nuclear export of mRNA".RNA Biology.6(2): 149–152.doi:10.4161/rna.6.2.8046.PMID19229134.
- ^Cenik C, Chua HN, Zhang H, Tarnawsky SP, Akef A, Derti A, Tasan M, Moore MJ, Palazzo AF, Roth FP (April 2011)."Genome analysis reveals interplay between 5'UTR introns and nuclear mRNA export for secretory and mitochondrial genes".PLOS Genetics.7(4): e1001366.doi:10.1371/journal.pgen.1001366.PMC3077370.PMID21533221.
- ^Steward O, Levy WB (March 1982)."Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus".The Journal of Neuroscience.2(3): 284–291.doi:10.1523/JNEUROSCI.02-03-00284.1982.PMC6564334.PMID7062109.
- ^Steward O, Worley PF (April 2001)."Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation".Neuron.30(1): 227–240.doi:10.1016/s0896-6273(01)00275-6.PMID11343657.S2CID13395819.
- ^Job C,Eberwine J(December 2001). "Localization and translation of mRNA in dendrites and axons".Nature Reviews. Neuroscience.2(12): 889–898.doi:10.1038/35104069.PMID11733796.S2CID5275219.
- ^Oleynikov Y, Singer RH (February 2003)."Real-time visualization of ZBP1 association with beta-actin mRNA during transcription and localization".Current Biology.13(3): 199–207.Bibcode:2003CBio...13..199O.doi:10.1016/s0960-9822(03)00044-7.PMC4765734.PMID12573215.
- ^Hüttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, et al. (November 2005). "Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1".Nature.438(7067): 512–515.Bibcode:2005Natur.438..512H.doi:10.1038/nature04115.PMID16306994.S2CID2453397.
- ^Oleynikov Y, Singer RH (October 1998)."RNA localization: different zipcodes, same postman?".Trends in Cell Biology.8(10): 381–383.doi:10.1016/s0962-8924(98)01348-8.PMC2136761.PMID9789325.
- ^Ainger K, Avossa D, Diana AS, Barry C, Barbarese E, Carson JH (September 1997)."Transport and localization elements in myelin basic protein mRNA".The Journal of Cell Biology.138(5): 1077–1087.doi:10.1083/jcb.138.5.1077.PMC2136761.PMID9281585.
- ^Haimovich G, Ecker CM, Dunagin MC, Eggan E, Raj A, Gerst JE, Singer RH (November 2017)."Intercellular mRNA trafficking via membrane nanotube-like extensions in mammalian cells".Proceedings of the National Academy of Sciences of the United States of America.114(46): E9873–E9882.Bibcode:2017PNAS..114E9873H.doi:10.1073/pnas.1706365114.PMC5699038.PMID29078295.
- ^Haimovich G, Dasgupta S, Gerst JE (February 2021)."RNA transfer through tunneling nanotubes".Biochemical Society Transactions.49(1): 145–160.doi:10.1042/BST20200113.PMID33367488.S2CID229689880.
- ^Lin CY, Beattie A, Baradaran B, Dray E, Duijf PH (September 2018)."Contradictory mRNA and protein misexpression of EEF1A1 in ductal breast carcinoma due to cell cycle regulation and cellular stress".Scientific Reports.8(1): 13904.Bibcode:2018NatSR...813904L.doi:10.1038/s41598-018-32272-x.PMC6141510.PMID30224719.
- ^Shabalina SA, Ogurtsov AY, Spiridonov NA (2006)."A periodic pattern of mRNA secondary structure created by the genetic code".Nucleic Acids Research.34(8): 2428–2437.doi:10.1093/nar/gkl287.PMC1458515.PMID16682450.
- ^Katz L, Burge CB (September 2003)."Widespread selection for local RNA secondary structure in coding regions of bacterial genes".Genome Research.13(9): 2042–2051.doi:10.1101/gr.1257503.PMC403678.PMID12952875.
- ^Lu YF, Mauger DM, Goldstein DB, Urban TJ, Weeks KM, Bradrick SS (November 2015)."IFNL3 mRNA structure is remodeled by a functional non-coding polymorphism associated with hepatitis C virus clearance".Scientific Reports.5:16037.Bibcode:2015NatSR...516037L.doi:10.1038/srep16037.PMC4631997.PMID26531896.
- ^ab Kozak M (March 1983)."Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles".Microbiological Reviews.47(1): 1–45.doi:10.1128/MMBR.47.1.1-45.1983.PMC281560.PMID6343825.
- ^Niehrs C, Pollet N (December 1999). "Synexpression groups in eukaryotes".Nature.402(6761): 483–487.Bibcode:1999Natur.402..483N.doi:10.1038/990025.PMID10591207.S2CID4349134.
- ^Mercer TR, Neph S, Dinger ME, Crawford J, Smith MA, Shearwood AM, Haugen E, Bracken CP, Rackham O,Stamatoyannopoulos JA,Filipovska A, Mattick JS (August 2011)."The human mitochondrial transcriptome".Cell.146(4): 645–658.doi:10.1016/j.cell.2011.06.051.PMC3160626.PMID21854988.
- ^Wells SE, Hillner PE, Vale RD, Sachs AB (July 1998). "Circularization of mRNA by eukaryotic translation initiation factors".Molecular Cell.2(1): 135–140.CiteSeerX10.1.1.320.5704.doi:10.1016/S1097-2765(00)80122-7.PMID9702200.
- ^López-Lastra M, Rivas A, Barría MI (2005)."Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation".Biological Research.38(2–3): 121–146.doi:10.4067/S0716-97602005000200003.PMID16238092.
- ^Zhang X, Liang Z, Wang C, Shen Z, Sun S, Gong C, Hu X (2022)."Viral Circular RNAs and Their Possible Roles in Virus-Host Interaction".Frontiers in Immunology.13:939768.doi:10.3389/fimmu.2022.939768.PMC9247149.PMID35784275.
- ^Lewin B,Krebs JE, Kilpatrick ST, Goldstein ES, eds. (2011).Lewin's genes X(10th ed.). Sudbury, Mass.: Jones and Bartlett.ISBN9780763766320.OCLC456641931.
- ^Yu J, Russell JE (September 2001)."Structural and functional analysis of an mRNP complex that mediates the high stability of human beta-globin mRNA".Molecular and Cellular Biology.21(17): 5879–5888.doi:10.1128/mcb.21.17.5879-5888.2001.PMC87307.PMID11486027.
- ^Deana A, Celesnik H, Belasco JG (January 2008). "The bacterial enzyme RppH triggers messenger RNA degradation by 5' pyrophosphate removal".Nature.451(7176): 355–358.Bibcode:2008Natur.451..355D.doi:10.1038/nature06475.PMID18202662.S2CID4321451.
- ^Parker R, Sheth U (March 2007)."P bodies and the control of mRNA translation and degradation".Molecular Cell.25(5): 635–646.doi:10.1016/j.molcel.2007.02.011.PMID17349952.
- ^Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M (November 2001)."AU binding proteins recruit the exosome to degrade ARE-containing mRNAs".Cell.107(4): 451–464.doi:10.1016/S0092-8674(01)00578-5.PMID11719186.S2CID14817671.
- ^Fenger-Grøn M, Fillman C, Norrild B, Lykke-Andersen J (December 2005)."Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping".Molecular Cell.20(6): 905–915.doi:10.1016/j.molcel.2005.10.031.PMID16364915.
- ^Shaw G, Kamen R (August 1986). "A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation".Cell.46(5): 659–667.doi:10.1016/0092-8674(86)90341-7.PMID3488815.S2CID40332253.
- ^Chen CY, Shyu AB (November 1995). "AU-rich elements: characterization and importance in mRNA degradation".Trends in Biochemical Sciences.20(11): 465–470.doi:10.1016/S0968-0004(00)89102-1.PMID8578590.
- ^Isken O, Maquat LE (August 2007)."Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function".Genes & Development.21(15): 1833–1856.doi:10.1101/gad.1566807.PMID17671086.
- ^Obbard DJ, Gordon KH, Buck AH, Jiggins FM (January 2009)."The evolution of RNAi as a defence against viruses and transposable elements".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.364(1513): 99–115.doi:10.1098/rstb.2008.0168.PMC2592633.PMID18926973.
- ^Robert E. Farrell, Jr. RNA Methodologies, 5th Edition. Academic Press, 2017
- ^Brennecke J, Stark A, Russell RB, Cohen SM (March 2005)."Principles of microRNA-target recognition".PLOS Biology.3(3): e85.doi:10.1371/journal.pbio.0030085.PMC1043860.PMID15723116.
- ^Tasuku Honjo, Michael Reth, Andreas Radbruch, Frederick Alt. Molecular Biology of B Cells, 2nd Edition. Academic Press, 2014 (including "updated research on microRNAs" )
- ^Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E (January 2009)."Deadenylation is a widespread effect of miRNA regulation".RNA.15(1): 21–32.doi:10.1261/rna.1399509.PMC2612776.PMID19029310.
- ^abHajj KA, Whitehead KA (12 September 2017)."Tools for translation: non-viral materials for therapeutic mRNA delivery".Nature Reviews Materials.2(10): 17056.Bibcode:2017NatRM...217056H.doi:10.1038/natrevmats.2017.56.
- ^abGousseinov E, Kozlov M, Scanlan C (September 15, 2015)."RNA-Based Therapeutics and Vaccines".Genetic Engineering News.
- ^Kaczmarek JC, Kowalski PS, Anderson DG (June 2017)."Advances in the delivery of RNA therapeutics: from concept to clinical reality".Genome Medicine.9(1): 60.doi:10.1186/s13073-017-0450-0.PMC5485616.PMID28655327.
- ^Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ (November 2012)."Developing mRNA-vaccine technologies".RNA Biology.9(11): 1319–30.doi:10.4161/rna.22269.PMC3597572.PMID23064118.
- ^Haridi R (2021-04-23)."The mRNA revolution: How COVID-19 hit fast-forward on an experimental technology".New Atlas.Retrieved2021-04-26.
- ^Kowalska J, Wypijewska del Nogal A, Darzynkiewicz ZM, Buck J, Nicola C, Kuhn AN, Lukaszewicz M, Zuberek J, Strenkowska M, Ziemniak M, Maciejczyk M, Bojarska E, Rhoads RE, Darzynkiewicz E, Sahin U, Jemielity J (2014), "mRNA-based therapeutics–developing a new class of drugs.",Nature Reviews Drug Discovery,vol. 13, no. 10, pp. 759–780,doi:10.1093/nar/gku757,PMC4176373,PMID25150148
- ^Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG (June 2022)."The clinical progress of mRNA vaccines and immunotherapies".Nature Biotechnology.40(6): 840–854.doi:10.1038/s41587-022-01294-2.PMID35534554.S2CID248667843.
- ^"The Nobel Prize in Physiology or Medicine 2023".NobelPrize.org.Retrieved2023-10-03.
- ^"Hungarian and US scientists win Nobel for COVID-19 vaccine discoveries".Reuters.2023-10-02.Retrieved2023-10-03.
- ^"The Nobel Prize in Physiology or Medicine 2023".NobelPrize.org.Retrieved2023-10-03.
- ^Monod J, Pappenheimer AM, Cohen-Bazire G (1952). "La cinétique de la biosynthèse de la β-galactosidase chez E. coli considérée comme fonction de la croissance".Biochimica et Biophysica Acta(in French).9(6): 648–660.doi:10.1016/0006-3002(52)90227-8.PMID13032175.
- ^Pardee AB (May 1954)."Nucleic Acid Precursors and Protein Synthesis".Proceedings of the National Academy of Sciences of the United States of America.40(5): 263–270.Bibcode:1954PNAS...40..263P.doi:10.1073/pnas.40.5.263.PMC534118.PMID16589470.
- ^Hershey AD, Dixon J, Chase M (July 1953)."Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition".The Journal of General Physiology.36(6): 777–789.doi:10.1085/jgp.36.6.777.PMC2147416.PMID13069681.
- ^abcCobb M(29 June 2015)."Who discovered messenger RNA?".Current Biology.25(13): R526–R532.Bibcode:2015CBio...25.R526C.doi:10.1016/j.cub.2015.05.032.PMID26126273.
Further reading
[edit]- Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F (February 2016)."Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk".Scientific Reports.6(1): 20680.Bibcode:2016NatSR...620680A.doi:10.1038/srep20680.PMC4745068.PMID26854194.
- Lillycrop KA,Burdge GC (October 2012). "Epigenetic mechanisms linking early nutrition to long term health".Best Practice & Research. Clinical Endocrinology & Metabolism.26(5): 667–676.doi:10.1016/j.beem.2012.03.009.PMID22980048.
- Melnik BC, Kakulas F, Geddes DT, Hartmann PE, John SM, Carrera-Bastos P, Cordain L, Schmitz G (21 June 2016)."Milk miRNAs: simple nutrients or systemic functional regulators?".Nutrition & Metabolism.13(1): 42.doi:10.1186/s12986-016-0101-2.PMC4915038.PMID27330539.
- Vickers MH (June 2014)."Early life nutrition, epigenetics and programming of later life disease".Nutrients.6(6): 2165–2178.doi:10.3390/nu6062165.PMC4073141.PMID24892374.
- Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X (2012)."Immune-related microRNAs are abundant in breast milk exosomes".International Journal of Biological Sciences.8(1): 118–123.doi:10.7150/ijbs.8.118.PMC3248653.PMID22211110.
- Krause W (2023)."mRNA — From COVID-19 Treatment to Cancer Immunotherapy".Biomedicines.11(2): 308.doi:10.3390/biomedicines11020308.PMC9953480.PMID36830845.
External links
[edit]- RNAi Atlas:a database of RNAi libraries and their target analysis results
- miRSearchArchived2012-12-04 at theWayback Machine:Tool for finding microRNAs that target mRNA
- How mRNA is coded?:YouTube video
- What is mRNA?:theconversation.com
