Macrocycle
It has been suggested thatMacrocyclic stereocontrolbemergedinto this article. (Discuss)Proposed since January 2024. |

Macrocyclesare often described as molecules and ions containing aringof twelve or more atoms. Classical examples include thecrown ethers,calixarenes,porphyrins,andcyclodextrins.Macrocycles describe a large, mature area of chemistry.[2]
Macrocycle:Cyclic macromolecule or a macromolecular cyclic portion of a macromolecule. Note 1: A cyclic macromolecule has no end-groups but may nevertheless be regarded as a chain.
Note 2: In the literature, the term macrocycle is sometimes used for molecules of low relative molecular mass that would not be considered macromolecules.[3]
Synthesis[edit]
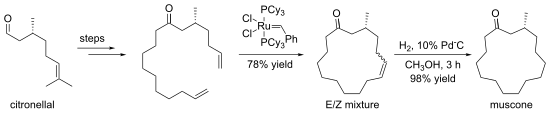
The formation of macrocycles by ring-closure is calledmacrocylization.[4]Pioneering work was reported for studies onterpenoidmacrocycles.[5]The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by usinghigh-dilution reactions,whereby intramolecular processes are favored relative to polymerizations.[6]
Some macrocyclizations are favored usingtemplate reactions.Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size.[7]Thecrown ethersare often generated in the presence of an alkali metal cation, which organizes the condensing components by complexation.[8]An illustrative macrocyclization is the synthesis of (−)-musconefrom (+)-citronellal.The 15-membered ring is generated byring-closing metathesis.[9]
Occurrence and applications[edit]
One important application are the many macrocyclic antibiotics, themacrolides,e.g.clarithromycin.Many metallocofactors are bound to macrocyclic ligands, which includeporphyrins,corrins,andchlorins.These rings arise from multistep biosynthetic processes that also feature macrocycles.
Macrocycles often bind ions and facilitateion transportacross hydrophobic membranes and solvents. The macrocycle envelops the ion with a hydrophobic sheath, which facilitatesphase transferproperties.[11]

Macrocycles are often bioactive and could be useful for drug delivery.[12][13]
Subdivisions[edit]
See also[edit]
References[edit]
- ^Hamilton-Miller, JM (1973)."Chemistry and Biology of the Polyene Macrolide Antibiotics".Bacteriological Reviews.37(2): 166–196.doi:10.1128/br.37.2.166-196.1973.PMC413810.PMID4578757.
- ^Zhichang Liu; Siva Krishna Mohan Nalluria; J. Fraser Stoddart (2017). "Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes".Chemical Society Reviews.46(9): 2459–2478.doi:10.1039/c7cs00185a.PMID28462968.
- ^R. G. Jones; J. Kahovec; R. Stepto; E. S. Wilks; M. Hess; T. Kitayama; W. V. Metanomski (2008).IUPAC. Compendium of Polymer Terminology and Nomenclature, IUPAC Recommendations 2008 (the "Purple Book" )(PDF).RSC Publishing, Cambridge, UK.
- ^François Diederich; Peter J. Stang; Rik R. Tykwinski, eds. (2008).Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis.Wiley-VCH.doi:10.1002/9783527621484.ISBN9783527621484.
- ^H. Höcker (2009)."Cyclic and Macrocyclic OrganicCompounds – a Personal Review in Honor of Professor Leopold Ružička".Cyclic and Macrocyclic Organic Compounds, Kem. Ind.58:73–80.
- ^Vicente Martí-Centelles; Mrituanjay D. Pandey; M. Isabel Burguete; Santiago V. Luis (2015)."Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization".Chem. Rev.115(16): 8736–8834.doi:10.1021/acs.chemrev.5b00056.hdl:10234/154905.PMID26248133.
- ^Gerbeleu, Nicolai V.; Arion, Vladimir B.; Burgess, John (2007). Nicolai V. Gerbeleu; Vladimir B. Arion; John Burgess (eds.).Template Synthesis of Macrocyclic Compounds.Wiley-VCH.doi:10.1002/9783527613809.ISBN9783527613809.
- ^Pedersen, Charles J. (1988)."Macrocyclic Polyethers: Dibenzo-18-Crown-6 Polyether and Dicyclohexyl-18-Crown-6 Polyether".Organic Syntheses;Collected Volumes,vol. 6, p. 395.
- ^Kamat, V.P.; Hagiwara, H.; Katsumi, T.; Hoshi, T.; Suzuki, T.; Ando, M. (2000). "Ring Closing Metathesis Directed Synthesis of (R)-(−)-Muscone from (+)-Citronellal".Tetrahedron.56(26): 4397–4403.doi:10.1016/S0040-4020(00)00333-1.
- ^Paul R. Ortiz de Montellano (2008). "Hemes in Biology".Wiley Encyclopedia of Chemical Biology.John Wiley & Sons. pp. 1–10.doi:10.1002/9780470048672.wecb221.ISBN978-0470048672.
- ^Choi, Kihang; Hamilton, Andrew D. (2003). "Macrocyclic anion receptors based on directed hydrogen bonding interactions".Coordination Chemistry Reviews.240(1–2): 101–110.doi:10.1016/s0010-8545(02)00305-3.
- ^Ermert, Philipp (2017-10-25)."Design, Properties and Recent Application of Macrocycles in Medicinal Chemistry".CHIMIA International Journal for Chemistry.71(10): 678–702.doi:10.2533/chimia.2017.678.PMID29070413.
- ^Marsault, Eric; Peterson, Mark L. (2011-04-14). "Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery".Journal of Medicinal Chemistry.54(7): 1961–2004.doi:10.1021/jm1012374.ISSN0022-2623.PMID21381769.
Further reading[edit]
- Chambron, J-C.; Dietrich-Buchecker, C.; Hemmert, C.; Khemiss, A-K.; Mitchell, D.; Sauvage, J-P.; Weiss, J. (1990)."Interlacing molecular threads on transition metals"(PDF).Pure Appl. Chem.62(6): 1027–34.doi:10.1351/pac199062061027.S2CID21741762.
- Iyoda, Masahiko; Yamakawa, Jun; Rahman, M. Jalilur (2011-11-04). "Conjugated Macrocycles: Concepts and Applications".Angewandte Chemie International Edition.50(45): 10522–10553.doi:10.1002/anie.201006198.ISSN1521-3773.PMID21960431.