Metabolism

| Part of a series on |
| Biochemistry |
|---|
 |
Metabolism(/məˈtæbəlɪzəm/,fromGreek:μεταβολήmetabolē,"change" ) is the set oflife-sustainingchemical reactionsinorganisms.The three main functions of metabolism are: the conversion of the energy in food toenergyavailable to run cellular processes; the conversion of food to building blocks ofproteins,lipids,nucleic acids,and somecarbohydrates;and the elimination ofmetabolic wastes.Theseenzyme-catalyzed reactions allow organisms to grow and reproduce, maintain theirstructures,and respond to their environments. The wordmetabolismcan also refer to the sum of all chemical reactions that occur in living organisms, includingdigestionand the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism.
Metabolic reactions may be categorized ascatabolic—thebreaking downof compounds (for example, of glucose to pyruvate bycellular respiration); oranabolic—thebuilding up(synthesis) of compounds (such as proteins, carbohydrates, lipids, and nucleic acids). Usually, catabolism releases energy, and anabolism consumes energy.
The chemical reactions of metabolism are organized intometabolic pathways,in which one chemical is transformed through a series of steps into another chemical, each step being facilitated by a specificenzyme.Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that requireenergyand will not occur by themselves, bycouplingthem tospontaneous reactionsthat release energy. Enzymes act ascatalysts—they allow a reaction to proceed more rapidly—and they also allow theregulationof the rate of a metabolic reaction, for example in response to changes in thecell'senvironment or tosignalsfrom other cells.
The metabolic system of a particular organism determines which substances it will findnutritiousand whichpoisonous.For example, someprokaryotesusehydrogen sulfideas a nutrient, yet this gas is poisonous to animals.[1]Thebasal metabolic rateof an organism is the measure of the amount of energy consumed by all of these chemical reactions.
A striking feature of metabolism is the similarity of the basic metabolic pathways among vastly different species.[2]For example, the set ofcarboxylic acidsthat are best known as the intermediates in thecitric acid cycleare present in all known organisms, being found in species as diverse as theunicellularbacteriumEscherichia coliand hugemulticellular organismslikeelephants.[3]These similarities in metabolic pathways are likely due to their early appearance inevolutionary history,and their retention is likely due to theirefficacy.[4][5]In various diseases, such astype II diabetes,metabolic syndrome,andcancer,normal metabolism is disrupted.[6]The metabolism of cancer cells is also different from the metabolism of normal cells, and these differences can be used to find targets for therapeutic intervention in cancer.[7]
Key biochemicals
[edit]
Most of the structures that make up animals, plants and microbes are made from four basic classes ofmolecules:amino acids,carbohydrates,nucleic acidandlipids(often calledfats). As these molecules are vital for life, metabolic reactions either focus on making these molecules during the construction of cells and tissues, or on breaking them down and using them to obtain energy, by their digestion. These biochemicals can be joined to makepolymerssuch asDNAandproteins,essentialmacromoleculesof life.[8]
| Type of molecule | Name ofmonomerforms | Name ofpolymerforms | Examples of polymer forms |
|---|---|---|---|
| Amino acids | Amino acids | Proteins(made of polypeptides) | Fibrous proteinsandglobular proteins |
| Carbohydrates | Monosaccharides | Polysaccharides | Starch,glycogenandcellulose |
| Nucleic acids | Nucleotides | Polynucleotides | DNAandRNA |
Amino acids and proteins
[edit]Proteins are made ofamino acidsarranged in a linear chain joined bypeptide bonds.Many proteins areenzymesthatcatalyzethe chemical reactions in metabolism. Other proteins have structural or mechanical functions, such as those that form thecytoskeleton,a system ofscaffoldingthat maintains the cell shape.[9]Proteins are also important incell signaling,immune responses,cell adhesion,active transportacross membranes, and thecell cycle.[10]Amino acids also contribute to cellular energy metabolism by providing a carbon source for entry into the citric acid cycle (tricarboxylic acid cycle),[11]especially when a primary source of energy, such asglucose,is scarce, or when cells undergo metabolic stress.[12]
Lipids
[edit]Lipids are the most diverse group of biochemicals. Their main structural uses are as part of internal and externalbiological membranes,such as thecell membrane.[10]Theirchemical energycan also be used. Lipids contain a long, non-polarhydrocarbon chainwith a small polar region containing oxygen. Lipids are usually defined ashydrophobicoramphipathicbiological molecules but will dissolve inorganic solventssuch asethanol,benzeneorchloroform.[13]Thefatsare a large group of compounds that containfatty acidsandglycerol;a glycerol molecule attached to three fatty acids byesterlinkages is called atriacylglyceride.[14]Several variations of the basic structure exist, including backbones such assphingosineinsphingomyelin,andhydrophilicgroups such asphosphateinphospholipids.Steroidssuch assterolare another major class of lipids.[15]
Carbohydrates
[edit]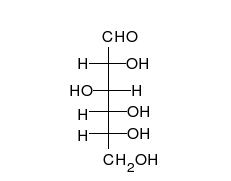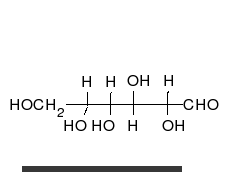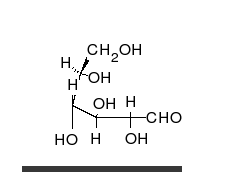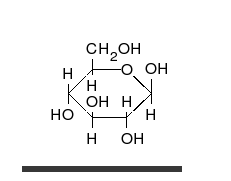
Carbohydrates arealdehydesorketones,with manyhydroxylgroups attached, that can exist as straight chains or rings. Carbohydrates are the most abundant biological molecules, and fill numerous roles, such as the storage and transport ofenergy(starch,glycogen) and structural components (cellulosein plants,chitinin animals).[10]The basic carbohydrate units are calledmonosaccharidesand includegalactose,fructose,and most importantlyglucose.Monosaccharides can be linked together to formpolysaccharidesin almost limitless ways.[16]
Nucleotides
[edit]The two nucleic acids, DNA andRNA,are polymers ofnucleotides.Each nucleotide is composed of a phosphate attached to ariboseordeoxyribosesugar group which is attached to anitrogenous base.Nucleic acids are critical for the storage and use of genetic information, and its interpretation through the processes oftranscriptionandprotein biosynthesis.[10]This information is protected byDNA repairmechanisms and propagated throughDNA replication.Manyviruseshave anRNA genome,such asHIV,which usesreverse transcriptionto create a DNA template from its viral RNA genome.[17]RNA inribozymessuch asspliceosomesandribosomesis similar to enzymes as it can catalyze chemical reactions. Individualnucleosidesare made by attaching anucleobaseto aribosesugar. These bases areheterocyclicrings containing nitrogen, classified aspurinesorpyrimidines.Nucleotides also act as coenzymes in metabolic-group-transfer reactions.[18]
Coenzymes
[edit]
Metabolism involves a vast array of chemical reactions, but most fall under a few basic types of reactions that involve the transfer offunctional groupsof atoms and their bonds within molecules.[19]This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions.[18]These group-transfer intermediates are calledcoenzymes.Each class of group-transfer reactions is carried out by a particular coenzyme, which is thesubstratefor a set of enzymes that produce it, and a set of enzymes that consume it. These coenzymes are therefore continuously made, consumed and then recycled.[20]
One central coenzyme isadenosine triphosphate(ATP), the energy currency of cells. Thisnucleotideis used to transfer chemical energy between different chemical reactions. There is only a small amount of ATP in cells, but as it is continuously regenerated, the human body can use about its own weight in ATP per day.[20]ATP acts as a bridge betweencatabolismandanabolism.Catabolism breaks down molecules, and anabolism puts them together. Catabolic reactions generate ATP, and anabolic reactions consume it. It also serves as a carrier of phosphate groups inphosphorylationreactions.[21]
Avitaminis an organic compound needed in small quantities that cannot be made in cells. Inhuman nutrition,most vitamins function as coenzymes after modification; for example, all water-soluble vitamins are phosphorylated or are coupled to nucleotides when they are used in cells.[22]Nicotinamide adenine dinucleotide(NAD+), a derivative of vitamin B3(niacin), is an important coenzyme that acts as a hydrogen acceptor. Hundreds of separate types ofdehydrogenasesremove electrons from their substrates andreduceNAD+into NADH. This reduced form of the coenzyme is then a substrate for any of thereductasesin the cell that need to transfer hydrogen atoms to their substrates.[23]Nicotinamide adenine dinucleotide exists in two related forms in the cell, NADH and NADPH. The NAD+/NADH form is more important in catabolic reactions, while NADP+/NADPH is used in anabolic reactions.[24]
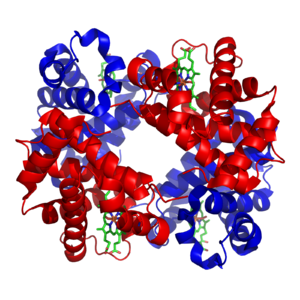
Mineral and cofactors
[edit]Inorganic elements play critical roles in metabolism; some are abundant (e.g.sodiumandpotassium) while others function at minute concentrations. About 99% of a human's body weight is made up of the elementscarbon,nitrogen,calcium,sodium,chlorine,potassium,hydrogen,phosphorus,oxygenandsulfur.Organic compounds(proteins, lipids and carbohydrates) contain the majority of the carbon and nitrogen; most of the oxygen and hydrogen is present as water.[25]
The abundant inorganic elements act aselectrolytes.The most important ions aresodium,potassium,calcium,magnesium,chloride,phosphateand the organic ionbicarbonate.The maintenance of preciseion gradientsacrosscell membranesmaintainsosmotic pressureandpH.[26]Ions are also critical fornerveandmusclefunction, asaction potentialsin these tissues are produced by the exchange of electrolytes between theextracellular fluidand the cell's fluid, thecytosol.[27]Electrolytes enter and leave cells through proteins in the cell membrane calledion channels.For example,muscle contractiondepends upon the movement of calcium, sodium and potassium through ion channels in the cell membrane andT-tubules.[28]
Transition metalsare usually present astrace elementsin organisms, withzincandironbeing most abundant of those.[29]Metal cofactors are bound tightly to specific sites in proteins; although enzyme cofactors can be modified during catalysis, they always return to their original state by the end of the reaction catalyzed. Metal micronutrients are taken up into organisms by specific transporters and bind to storage proteins such asferritinormetallothioneinwhen not in use.[30][31]
Catabolism
[edit]Catabolism is the set of metabolic processes that break down large molecules. These include breaking down and oxidizing food molecules. The purpose of the catabolic reactions is to provide the energy and components needed by anabolic reactions which build molecules.[32]The exact nature of these catabolic reactions differ from organism to organism, and organisms can be classified based on their sources of energy, hydrogen, and carbon (theirprimary nutritional groups), as shown in the table below. Organic molecules are used as a source of hydrogen atoms or electrons byorganotrophs,whilelithotrophsuse inorganic substrates. Whereasphototrophsconvert sunlight tochemical energy,[33]chemotrophsdepend onredoxreactions that involve the transfer of electrons from reduced donor molecules such asorganic molecules,hydrogen,hydrogen sulfideorferrous ionstooxygen,nitrateorsulfate.In animals, these reactions involve complexorganic moleculesthat are broken down to simpler molecules, such ascarbon dioxideand water.Photosyntheticorganisms, such as plants andcyanobacteria,use similar electron-transfer reactions to store energy absorbed from sunlight.[34]
| Energy source | sunlight | photo- | -troph | ||
| molecules | chemo- | ||||
| Hydrogen or electron donor | organic compound | organo- | |||
| inorganic compound | litho- | ||||
| Carbon source | organic compound | hetero- | |||
| inorganic compound | auto- | ||||
The most common set of catabolic reactions in animals can be separated into three main stages. In the first stage, large organic molecules, such asproteins,polysaccharidesorlipids,are digested into their smaller components outside cells. Next, these smaller molecules are taken up by cells and converted to smaller molecules, usuallyacetyl coenzyme A(acetyl-CoA), which releases some energy. Finally, the acetyl group on acetyl-CoA is oxidized to water and carbon dioxide in thecitric acid cycleandelectron transport chain,releasing more energy while reducing the coenzymenicotinamide adenine dinucleotide(NAD+) into NADH.[32]
Digestion
[edit]Macromolecules cannot be directly processed by cells. Macromolecules must be broken into smaller units before they can be used in cell metabolism. Different classes of enzymes are used to digest these polymers. Thesedigestive enzymesincludeproteasesthat digest proteins into amino acids, as well asglycoside hydrolasesthat digest polysaccharides into simple sugars known asmonosaccharides.[36]
Microbes simply secrete digestive enzymes into their surroundings,[37][38]while animals only secrete these enzymes from specialized cells in theirguts,including thestomachandpancreas,and insalivary glands.[39]The amino acids or sugars released by these extracellular enzymes are then pumped into cells byactive transportproteins.[40][41]

Energy from organic compounds
[edit]Carbohydrate catabolism is the breakdown of carbohydrates into smaller units. Carbohydrates are usually taken into cells after they have been digested intomonosaccharidessuch asglucoseandfructose.[42]Once inside, the major route of breakdown isglycolysis,in which glucose is converted intopyruvate.This process generates the energy-conveying moleculeNADHfrom NAD+,and generatesATPfromADPfor use in powering many processes within the cell.[43]Pyruvate is an intermediate in several metabolic pathways, but the majority is converted toacetyl-CoAand fed into thecitric acid cycle,which enables more ATP production by means ofoxidative phosphorylation.This oxidation consumes molecular oxygen and releases water and the waste product carbon dioxide. When oxygen is lacking, or when pyruvate is temporarily produced faster than it can be consumed by the citric acid cycle (as in intense muscular exertion), pyruvate is converted tolactateby the enzymelactate dehydrogenase,a process that also oxidizes NADH back to NAD+for re-use in further glycolysis, allowing energy production to continue.[44]The lactate is later converted back to pyruvate for ATP production where energy is needed, or back to glucose in theCori cycle.An alternative route for glucose breakdown is thepentose phosphate pathway,which produces less energy but supportsanabolism(biomolecule synthesis). This pathway reduces the coenzymeNADP+to NADPH and producespentosecompounds such asribose 5-phosphatefor synthesis of many biomolecules such asnucleotidesandaromatic amino acids.[45]

Fats are catabolized byhydrolysisto free fatty acids and glycerol. The glycerol enters glycolysis and the fatty acids are broken down bybeta oxidationto release acetyl-CoA, which then is fed into the citric acid cycle. Fatty acids release more energy upon oxidation than carbohydrates. Steroids are also broken down by some bacteria in a process similar to beta oxidation, and this breakdown process involves the release of significant amounts of acetyl-CoA, propionyl-CoA, and pyruvate, which can all be used by the cell for energy.M. tuberculosiscan also grow on the lipidcholesterolas a sole source of carbon, and genes involved in the cholesterol-use pathway(s) have been validated as important during various stages of the infection lifecycle ofM. tuberculosis.[46]
Amino acidsare either used to synthesize proteins and other biomolecules, or oxidized toureaand carbon dioxide to produce energy.[47]The oxidation pathway starts with the removal of the amino group by atransaminase.The amino group is fed into theurea cycle,leaving a deaminated carbon skeleton in the form of aketo acid.Several of these keto acids are intermediates in the citric acid cycle, for example α-ketoglutarateformed by deamination ofglutamate.[48]Theglucogenic amino acidscan also be converted into glucose, throughgluconeogenesis.[49]
Energy transformations
[edit]Oxidative phosphorylation
[edit]In oxidative phosphorylation, the electrons removed from organic molecules in areas such as the citric acid cycle are transferred to oxygen and the energy released is used to make ATP. This is done ineukaryotesby a series of proteins in the membranes of mitochondria called theelectron transport chain.Inprokaryotes,these proteins are found in the cell'sinner membrane.[50]These proteins use the energy fromreducedmolecules like NADH to pumpprotonsacross a membrane.[51]
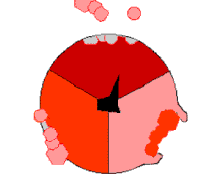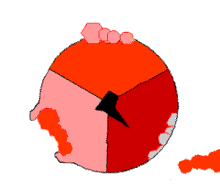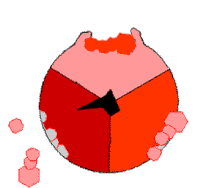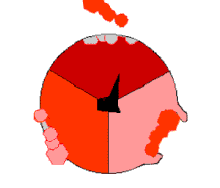
Pumping protons out of the mitochondria creates a protonconcentration differenceacross the membrane and generates anelectrochemical gradient.[52]This force drives protons back into the mitochondrion through the base of an enzyme calledATP synthase.The flow of protons makes the stalk subunit rotate, causing theactive siteof the synthase domain to change shape and phosphorylateadenosine diphosphate—turning it into ATP.[20]
Energy from inorganic compounds
[edit]Chemolithotrophyis a type of metabolism found inprokaryoteswhere energy is obtained from the oxidation ofinorganic compounds.These organisms can usehydrogen,[53]reducedsulfurcompounds (such assulfide,hydrogen sulfideandthiosulfate),[1]ferrous iron (Fe(II))[54]orammonia[55]as sources of reducing power and they gain energy from the oxidation of these compounds.[56]These microbial processes are important in globalbiogeochemical cyclessuch asacetogenesis,nitrificationanddenitrificationand are critical forsoil fertility.[57][58]
Energy from light
[edit]The energy in sunlight is captured byplants,cyanobacteria,purple bacteria,green sulfur bacteriaand someprotists.This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below. The energy capture and carbon fixation systems can, however, operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds.[59][60]
In many organisms, the capture of solar energy is similar in principle to oxidative phosphorylation, as it involves the storage of energy as a proton concentration gradient. This proton motive force then drives ATP synthesis.[61]The electrons needed to drive this electron transport chain come from light-gathering proteins calledphotosynthetic reaction centres.Reaction centers are classified into two types depending on the nature ofphotosynthetic pigmentpresent, with most photosynthetic bacteria only having one type, while plants and cyanobacteria have two.[62]
In plants, algae, and cyanobacteria,photosystem IIuses light energy to remove electrons from water, releasing oxygen as a waste product. The electrons then flow to thecytochrome b6f complex,which uses their energy to pump protons across thethylakoidmembrane in thechloroplast.[34]These protons move back through the membrane as they drive the ATP synthase, as before. The electrons then flow throughphotosystem Iand can then be used to reduce the coenzyme NADP+.[63]This coenzyme can enter theCalvin cycleor be recycled for further ATP generation.[citation needed]
Anabolism
[edit]Anabolismis the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from smaller and simpler precursors. Anabolism involves three basic stages. First, the production of precursors such asamino acids,monosaccharides,isoprenoidsandnucleotides,secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such asproteins,polysaccharides,lipidsandnucleic acids.[64]
Anabolism in organisms can be different according to the source of constructed molecules in their cells.Autotrophssuch as plants can construct the complex organic molecules in their cells such as polysaccharides and proteins from simple molecules likecarbon dioxideand water.Heterotrophs,on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from oxidation reactions.[64]
Carbon fixation
[edit]
Photosynthesis is the synthesis of carbohydrates from sunlight andcarbon dioxide(CO2). In plants, cyanobacteria and algae, oxygenic photosynthesis splits water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by thephotosynthetic reaction centres,as described above, to convert CO2intoglycerate 3-phosphate,which can then be converted into glucose. This carbon-fixation reaction is carried out by the enzymeRuBisCOas part of theCalvin–Benson cycle.[65]Three types of photosynthesis occur in plants,C3 carbon fixation,C4 carbon fixationandCAM photosynthesis.These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO2directly, while C4 and CAM photosynthesis incorporate the CO2into other compounds first, as adaptations to deal with intense sunlight and dry conditions.[66]
In photosyntheticprokaryotesthe mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin–Benson cycle, areversed citric acidcycle,[67]or thecarboxylationof acetyl-CoA.[68][69]Prokaryoticchemoautotrophsalso fix CO2through the Calvin–Benson cycle, but use energy from inorganic compounds to drive the reaction.[70]
Carbohydrates and glycans
[edit]In carbohydrate anabolism, simple organic acids can be converted intomonosaccharidessuch asglucoseand then used to assemblepolysaccharidessuch asstarch.The generation ofglucosefrom compounds likepyruvate,lactate,glycerol,glycerate 3-phosphateandamino acidsis calledgluconeogenesis.Gluconeogenesis converts pyruvate toglucose-6-phosphatethrough a series of intermediates, many of which are shared withglycolysis.[43]However, this pathway is not simplyglycolysisrun in reverse, as several steps are catalyzed by non-glycolytic enzymes. This is important as it allows the formation and breakdown of glucose to be regulated separately, and prevents both pathways from running simultaneously in afutile cycle.[71][72]
Although fat is a common way of storing energy, invertebratessuch as humans thefatty acidsin these stores cannot be converted to glucose throughgluconeogenesisas these organisms cannot convert acetyl-CoA intopyruvate;plants do, but animals do not, have the necessary enzymatic machinery.[73]As a result, after long-term starvation, vertebrates need to produceketone bodiesfrom fatty acids to replace glucose in tissues such as the brain that cannot metabolize fatty acids.[74]In other organisms such as plants and bacteria, this metabolic problem is solved using theglyoxylate cycle,which bypasses thedecarboxylationstep in the citric acid cycle and allows the transformation of acetyl-CoA tooxaloacetate,where it can be used for the production of glucose.[73][75]Other than fat, glucose is stored in most tissues, as an energy resource available within the tissue through glycogenesis which was usually being used to maintained glucose level in blood.[76]
Polysaccharides andglycansare made by the sequential addition of monosaccharides byglycosyltransferasefrom a reactive sugar-phosphate donor such asuridine diphosphate glucose(UDP-Glc) to an acceptorhydroxylgroup on the growing polysaccharide. As any of thehydroxylgroups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures.[77]The polysaccharides produced can have structural or metabolic functions themselves, or be transferred to lipids and proteins by the enzymesoligosaccharyltransferases.[78][79]
Fatty acids, isoprenoids and sterol
[edit]
Fatty acids are made byfatty acid synthasesthat polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acyl group, reduce it to an alcohol,dehydrateit to analkenegroup and then reduce it again to analkanegroup. The enzymes of fatty acid biosynthesis are divided into two groups: in animals and fungi, all these fatty acid synthase reactions are carried out by a single multifunctional type I protein,[80]while in plantplastidsand bacteria separate type II enzymes perform each step in the pathway.[81][82]
Terpenesandisoprenoidsare a large class of lipids that include thecarotenoidsand form the largest class of plantnatural products.[83]These compounds are made by the assembly and modification ofisopreneunits donated from the reactive precursorsisopentenyl pyrophosphateanddimethylallyl pyrophosphate.[84]These precursors can be made in different ways. In animals and archaea, themevalonate pathwayproduces these compounds from acetyl-CoA,[85]while in plants and bacteria thenon-mevalonate pathwayuses pyruvate andglyceraldehyde 3-phosphateas substrates.[84][86]One important reaction that uses these activated isoprene donors issterol biosynthesis.Here, the isoprene units are joined to makesqualeneand then folded up and formed into a set of rings to makelanosterol.[87]Lanosterol can then be converted into other sterols such ascholesterolandergosterol.[87][88]
Proteins
[edit]Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all twenty, but mammals can only synthesize eleven nonessential amino acids, so nineessential amino acidsmust be obtained from food.[10]Some simpleparasites,such as the bacteriaMycoplasma pneumoniae,lack all amino acid synthesis and take their amino acids directly from their hosts.[89]All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen is provided byglutamateandglutamine.Nonessensial amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is thentransaminatedto form an amino acid.[90]
Amino acids are made into proteins by being joined in a chain ofpeptide bonds.Each different protein has a unique sequence of amino acid residues: this is itsprimary structure.Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to atransfer RNAmolecule through anesterbond. Thisaminoacyl-tRNAprecursor is produced in anATP-dependent reaction carried out by anaminoacyl tRNA synthetase.[91]This aminoacyl-tRNA is then a substrate for theribosome,which joins the amino acid onto the elongating protein chain, using the sequence information in amessenger RNA.[92]
Nucleotide synthesis and salvage
[edit]Nucleotides are made from amino acids, carbon dioxide andformic acidin pathways that require large amounts of metabolic energy.[93]Consequently, most organisms have efficient systems to salvage preformed nucleotides.[93][94]Purinesare synthesized asnucleosides(bases attached toribose).[95]Bothadenineandguanineare made from the precursor nucleosideinosinemonophosphate, which is synthesized using atoms from the amino acidsglycine,glutamine,andaspartic acid,as well asformatetransferred from thecoenzymetetrahydrofolate.Pyrimidines,on the other hand, are synthesized from the baseorotate,which is formed from glutamine and aspartate.[96]
Xenobiotics and redox metabolism
[edit]All organisms are constantly exposed to compounds that they cannot use as foods and that would be harmful if they accumulated in cells, as they have no metabolic function. These potentially damaging compounds are calledxenobiotics.[97]Xenobiotics such assynthetic drugs,natural poisonsandantibioticsare detoxified by a set of xenobiotic-metabolizing enzymes. In humans, these includecytochrome P450 oxidases,[98]UDP-glucuronosyltransferases,[99]andglutathioneS-transferases.[100]This system of enzymes acts in three stages to firstly oxidize the xenobiotic (phase I) and then conjugate water-soluble groups onto the molecule (phase II). The modified water-soluble xenobiotic can then be pumped out of cells and in multicellular organisms may be further metabolized before being excreted (phase III). Inecology,these reactions are particularly important in microbialbiodegradationof pollutants and thebioremediationofcontaminated landand oil spills.[101]Many of these microbial reactions are shared with multicellular organisms, but due to the incredible diversity of types of microbes these organisms are able to deal with a far wider range of xenobiotics than multicellular organisms, and can degrade evenpersistent organic pollutantssuch asorganochloridecompounds.[102]
A related problem foraerobic organismsisoxidative stress.[103]Here, processes includingoxidative phosphorylationand the formation ofdisulfide bondsduringprotein foldingproducereactive oxygen speciessuch ashydrogen peroxide.[104]These damaging oxidants are removed byantioxidantmetabolites such asglutathioneand enzymes such ascatalasesandperoxidases.[105][106]
Thermodynamics of living organisms
[edit]Living organisms must obey thelaws of thermodynamics,which describe the transfer of heat andwork.Thesecond law of thermodynamicsstates that in anyisolated system,the amount ofentropy(disorder) cannot decrease. Although living organisms' amazing complexity appears to contradict this law, life is possible as all organisms areopen systemsthat exchange matter and energy with their surroundings. Living systems are not inequilibrium,but instead aredissipative systemsthat maintain their state of high complexity by causing a larger increase in the entropy of their environments.[107]The metabolism of a cell achieves this by coupling thespontaneous processesof catabolism to the non-spontaneous processes of anabolism. Inthermodynamicterms, metabolism maintains order by creating disorder.[108]
Regulation and control
[edit]As the environments of most organisms are constantly changing, the reactions of metabolism must be finelyregulatedto maintain a constant set of conditions within cells, a condition calledhomeostasis.[109][110]Metabolic regulation also allows organisms to respond to signals and interact actively with their environments.[111]Two closely linked concepts are important for understanding how metabolic pathways are controlled. Firstly, theregulationof an enzyme in a pathway is how its activity is increased and decreased in response to signals. Secondly, thecontrolexerted by this enzyme is the effect that these changes in its activity have on the overall rate of the pathway (thefluxthrough the pathway).[112]For example, an enzyme may show large changes in activity (i.e. it is highly regulated) but if these changes have little effect on the flux of a metabolic pathway, then this enzyme is not involved in the control of the pathway.[113]
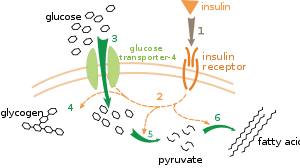
There are multiple levels of metabolic regulation. In intrinsic regulation, the metabolic pathway self-regulates to respond to changes in the levels of substrates or products; for example, a decrease in the amount of product can increase thefluxthrough the pathway to compensate.[112]This type of regulation often involvesallosteric regulationof the activities of multiple enzymes in the pathway.[114]Extrinsic control involves a cell in a multicellular organism changing its metabolism in response to signals from other cells. These signals are usually in the form of water-soluble messengers such ashormonesandgrowth factorsand are detected by specificreceptorson the cell surface.[115]These signals are then transmitted inside the cell bysecond messenger systemsthat often involved thephosphorylationof proteins.[116]
A very well understood example of extrinsic control is the regulation of glucose metabolism by the hormoneinsulin.[117]Insulin is produced in response to rises inblood glucose levels.Binding of the hormone toinsulin receptorson cells then activates a cascade ofprotein kinasesthat cause the cells to take up glucose and convert it into storage molecules such as fatty acids andglycogen.[118]The metabolism of glycogen is controlled by activity ofphosphorylase,the enzyme that breaks down glycogen, andglycogen synthase,the enzyme that makes it. These enzymes are regulated in a reciprocal fashion, with phosphorylation inhibiting glycogen synthase, but activating phosphorylase. Insulin causes glycogen synthesis by activatingprotein phosphatasesand producing a decrease in the phosphorylation of these enzymes.[119]
Evolution
[edit]
The central pathways of metabolism described above, such as glycolysis and the citric acid cycle, are present in allthree domainsof living things and were present in thelast universal common ancestor.[3][120]This universal ancestral cell wasprokaryoticand probably amethanogenthat had extensive amino acid, nucleotide, carbohydrate and lipid metabolism.[121][122]The retention of these ancient pathways during laterevolutionmay be the result of these reactions having been an optimal solution to their particular metabolic problems, with pathways such as glycolysis and the citric acid cycle producing their end products highly efficiently and in a minimal number of steps.[4][5]The first pathways of enzyme-based metabolism may have been parts ofpurinenucleotide metabolism, while previous metabolic pathways were a part of the ancientRNA world.[123]
Many models have been proposed to describe the mechanisms by which novel metabolic pathways evolve. These include the sequential addition of novel enzymes to a short ancestral pathway, the duplication and then divergence of entire pathways as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway.[124]The relative importance of these mechanisms is unclear, but genomic studies have shown that enzymes in a pathway are likely to have a shared ancestry, suggesting that many pathways have evolved in a step-by-step fashion with novel functions created from pre-existing steps in the pathway.[125]An alternative model comes from studies that trace the evolution of proteins' structures in metabolic networks, this has suggested that enzymes are pervasively recruited, borrowing enzymes to perform similar functions in different metabolic pathways (evident in theMANET database)[126]These recruitment processes result in an evolutionary enzymatic mosaic.[127]A third possibility is that some parts of metabolism might exist as "modules" that can be reused in different pathways and perform similar functions on different molecules.[128]
As well as the evolution of new metabolic pathways, evolution can also cause the loss of metabolic functions. For example, in someparasitesmetabolic processes that are not essential for survival are lost and preformed amino acids, nucleotides and carbohydrates may instead be scavenged from thehost.[129]Similar reduced metabolic capabilities are seen inendosymbioticorganisms.[130]
Investigation and manipulation
[edit]
Classically, metabolism is studied by areductionistapproach that focuses on a single metabolic pathway. Particularly valuable is the use ofradioactive tracersat the whole-organism, tissue and cellular levels, which define the paths from precursors to final products by identifying radioactively labelled intermediates and products.[131]The enzymes that catalyze these chemical reactions can then bepurifiedand theirkineticsand responses toinhibitorsinvestigated. A parallel approach is to identify the small molecules in a cell or tissue; the complete set of these molecules is called themetabolome.Overall, these studies give a good view of the structure and function of simple metabolic pathways, but are inadequate when applied to more complex systems such as the metabolism of a complete cell.[132]
An idea of the complexity of themetabolic networksin cells that contain thousands of different enzymes is given by the figure showing the interactions between just 43 proteins and 40 metabolites to the right: the sequences of genomes provide lists containing anything up to 26.500 genes.[133]However, it is now possible to use this genomic data to reconstruct complete networks of biochemical reactions and produce moreholisticmathematical models that may explain and predict their behavior.[134]These models are especially powerful when used to integrate the pathway and metabolite data obtained through classical methods with data ongene expressionfromproteomicandDNA microarraystudies.[135]Using these techniques, a model of human metabolism has now been produced, which will guide future drug discovery and biochemical research.[136]These models are now used innetwork analysis,to classify human diseases into groups that share common proteins or metabolites.[137][138]
Bacterial metabolic networks are a striking example ofbow-tie[139][140][141]organization, an architecture able to input a wide range of nutrients and produce a large variety of products and complex macromolecules using a relatively few intermediate common currencies.[142]
A major technological application of this information ismetabolic engineering.Here, organisms such asyeast,plants orbacteriaare genetically modified to make them more useful inbiotechnologyand aid the production ofdrugssuch asantibioticsor industrial chemicals such as1,3-propanediolandshikimic acid.[143][144][145]These genetic modifications usually aim to reduce the amount of energy used to produce the product, increase yields and reduce the production of wastes.[146]
History
[edit]The termmetabolismis derived from theAncient Greekword μεταβολή— "metabole" for "a change" which is derived from μεταβάλλειν— "metaballein", meaning "to change"[147]
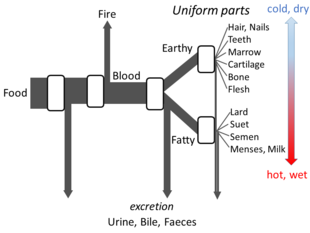
Greek philosophy
[edit]Aristotle'sThe Parts of Animalssets out enough details ofhis views on metabolismfor an open flow model to be made. He believed that at each stage of the process, materials from food were transformed, with heat being released as theclassical elementof fire, and residual materials being excreted as urine, bile, or faeces.[148]
Ibn al-Nafisdescribed metabolism in his 1260 AD work titledAl-Risalah al-Kamiliyyah fil Siera al-Nabawiyyah(The Treatise of Kamil on the Prophet's Biography) which included the following phrase "Both the body and its parts are in a continuous state of dissolution and nourishment, so they are inevitably undergoing permanent change."[149]
Application of the scientific method and Modern metabolic theories
[edit]The history of the scientific study of metabolism spans several centuries and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry. The first controlledexperimentsin human metabolism were published bySantorio Santorioin 1614 in his bookArs de statica medicina.[150]He described how he weighed himself before and after eating,sleep,working, sex, fasting, drinking, and excreting. He found that most of the food he took in was lost through what he called "insensible perspiration".

In these early studies, the mechanisms of these metabolic processes had not been identified and avital forcewas thought to animate living tissue.[151]In the 19th century, when studying thefermentationof sugar toalcoholbyyeast,Louis Pasteurconcluded that fermentation was catalyzed by substances within the yeast cells he called "ferments". He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells."[152]This discovery, along with the publication byFriedrich Wöhlerin 1828 of a paper on the chemical synthesis ofurea,[153]and is notable for being the first organic compound prepared from wholly inorganic precursors. This proved that the organic compounds and chemical reactions found in cells were no different in principle than any other part of chemistry.
It was the discovery ofenzymesat the beginning of the 20th century byEduard Buchnerthat separated the study of the chemical reactions of metabolism from the biological study of cells, and marked the beginnings ofbiochemistry.[154]The mass of biochemical knowledge grew rapidly throughout the early 20th century. One of the most prolific of these modern biochemists wasHans Krebswho made huge contributions to the study of metabolism.[155]He discovered the urea cycle and later, working withHans Kornberg,the citric acid cycle and the glyoxylate cycle.[156][157][75]Modern biochemical research has been greatly aided by the development of new techniques such aschromatography,X-ray diffraction,NMR spectroscopy,radioisotopic labelling,electron microscopyandmolecular dynamicssimulations. These techniques have allowed the discovery and detailed analysis of the many molecules and metabolic pathways in cells.[citation needed]
See also
[edit]- Anthropogenic metabolism– Material and energy turnover of human society
- Antimetabolite– Chemical that inhibits the use of a metabolite
- Calorimetry– Determining heat transfer in a system by measuring its other properties
- Isothermal microcalorimetry– Measuring versus elapsed time the net rate of heat flow
- Inborn errors of metabolism– Class of genetic diseases
- Iron–sulfur world hypothesis– Hypothetical scenario for the origin of life, a "metabolism first" theory of theorigin of life
- Metabolic disorder– Any disease hindering the body's ability to process and distribute nutrients
- Microphysiometry
- Primary nutritional groups– Group of organisms
- Proto-metabolism– Chemical reactions which turn into modern metabolism
- Respirometry– Estimation of metabolic rates by measuring heat production
- Stream metabolism
- Sulfur metabolism– Set of chemical reactions involving sulfur in living organisms
- Thermic effect of food– effect of food
- Urban metabolism– Model of the flows of materials and energy in cities
- Water metabolism– Aspect of homeostasis concerning control of the amount of water in an organism
- Overflow metabolism– Cellular phenomena
- Oncometabolism
- Reactome– Database of biological pathways
- KEGG– Collection of bioinformatics databases
References
[edit]- ^abFriedrich, CG (1997).Physiology and Genetics of Sulfur-oxidizing Bacteria.Advances in Microbial Physiology. Vol. 39. pp. 235–89.doi:10.1016/S0065-2911(08)60018-1.ISBN978-0-12-027739-1.PMID9328649.
- ^Pace NR (January 2001)."The universal nature of biochemistry".Proceedings of the National Academy of Sciences of the United States of America.98(3): 805–8.Bibcode:2001PNAS...98..805P.doi:10.1073/pnas.98.3.805.PMC33372.PMID11158550.
- ^abSmith E, Morowitz HJ (September 2004)."Universality in intermediary metabolism".Proceedings of the National Academy of Sciences of the United States of America.101(36): 13168–73.Bibcode:2004PNAS..10113168S.doi:10.1073/pnas.0404922101.PMC516543.PMID15340153.
- ^abEbenhöh O, Heinrich R (January 2001). "Evolutionary optimization of metabolic pathways. Theoretical reconstruction of the stoichiometry of ATP and NADH producing systems".Bulletin of Mathematical Biology.63(1): 21–55.doi:10.1006/bulm.2000.0197.PMID11146883.S2CID44260374.
- ^abMeléndez-Hevia E, Waddell TG, Cascante M (September 1996). "The puzzle of the Krebs citric acid cycle: assembling the pieces of chemically feasible reactions, and opportunism in the design of metabolic pathways during evolution".Journal of Molecular Evolution.43(3): 293–303.Bibcode:1996JMolE..43..293M.doi:10.1007/BF02338838.PMID8703096.S2CID19107073.
- ^Smith RL, Soeters MR, Wüst RC, Houtkooper RH (August 2018)."Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease".Endocrine Reviews.39(4): 489–517.doi:10.1210/er.2017-00211.PMC6093334.PMID29697773.
- ^Vander Heiden MG, DeBerardinis RJ (February 2017)."Understanding the Intersections between Metabolism and Cancer Biology".Cell.168(4): 657–669.doi:10.1016/j.cell.2016.12.039.PMC5329766.PMID28187287.
- ^Cooper GM (2000)."The Molecular Composition of Cells".The Cell: A Molecular Approach(2nd ed.).Archivedfrom the original on 27 August 2020.Retrieved25 June2020.
- ^Michie KA, Löwe J (2006). "Dynamic filaments of the bacterial cytoskeleton".Annual Review of Biochemistry.75:467–92.doi:10.1146/annurev.biochem.75.103004.142452.PMID16756499.S2CID4550126.
- ^abcdeNelson DL, Cox MM (2005).Lehninger Principles of Biochemistry.New York: W. H. Freeman and company. p.841.ISBN978-0-7167-4339-2.
- ^Kelleher JK, Bryan BM, Mallet RT, Holleran AL, Murphy AN, Fiskum G (September 1987)."Analysis of tricarboxylic acid-cycle metabolism of hepatoma cells by comparison of 14CO2 ratios".The Biochemical Journal.246(3): 633–9.doi:10.1042/bj2460633.PMC1148327.PMID3120698.
- ^Hothersall JS, Ahmed A (2013)."Metabolic fate of the increased yeast amino Acid uptake subsequent to catabolite derepression".Journal of Amino Acids.2013:461901.doi:10.1155/2013/461901.PMC3575661.PMID23431419.
- ^Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Murphy RC, et al. (May 2005)."A comprehensive classification system for lipids".Journal of Lipid Research.46(5): 839–61.doi:10.1194/jlr.E400004-JLR200.PMID15722563.
- ^"Lipid nomenclature Lip-1 & Lip-2".qmul.ac.uk.Archivedfrom the original on 6 June 2020.Retrieved6 June2020.
- ^Berg JM, Tymoczko JL, Gatto Jr GJ, Stryer L (8 April 2015).Biochemistry(8 ed.). New York: W. H. Freeman. p. 362.ISBN978-1-4641-2610-9.OCLC913469736.
- ^Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R (November 2005). "Glycomics: an integrated systems approach to structure-function relationships of glycans".Nature Methods.2(11): 817–24.doi:10.1038/nmeth807.PMID16278650.S2CID4644919.
- ^Sierra S, Kupfer B, Kaiser R (December 2005). "Basics of the virology of HIV-1 and its replication".Journal of Clinical Virology.34(4): 233–44.doi:10.1016/j.jcv.2005.09.004.PMID16198625.
- ^abWimmer MJ, Rose IA (1978). "Mechanisms of enzyme-catalyzed group transfer reactions".Annual Review of Biochemistry.47:1031–78.doi:10.1146/annurev.bi.47.070178.005123.PMID354490.
- ^Mitchell P (March 1979)."The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems".European Journal of Biochemistry.95(1): 1–20.doi:10.1111/j.1432-1033.1979.tb12934.x.PMID378655.
- ^abcDimroth P, von Ballmoos C, Meier T (March 2006)."Catalytic and mechanical cycles in F-ATP synthases. Fourth in the Cycles Review Series".EMBO Reports.7(3): 276–82.doi:10.1038/sj.embor.7400646.PMC1456893.PMID16607397.
- ^Bonora M, Patergnani S, Rimessi A, De Marchi E, Suski JM, Bononi A, et al. (September 2012)."ATP synthesis and storage".Purinergic Signalling.8(3): 343–57.doi:10.1007/s11302-012-9305-8.PMC3360099.PMID22528680.
- ^Berg JM, Tymoczko JL, Stryer L (2002)."Vitamins Are Often Precursors to Coenzymes".Biochemistry. 5th Edition.Archivedfrom the original on 15 December 2020.Retrieved9 June2020.
- ^Pollak N, Dölle C, Ziegler M (March 2007)."The power to reduce: pyridine nucleotides--small molecules with a multitude of functions".The Biochemical Journal.402(2): 205–18.doi:10.1042/BJ20061638.PMC1798440.PMID17295611.
- ^Fatih Y (2009).Advances in food biochemistry.Boca Raton: CRC Press. p. 228.ISBN978-1-4200-0769-5.OCLC607553259.
- ^Heymsfield SB, Waki M, Kehayias J, Lichtman S, Dilmanian FA, Kamen Y, et al. (August 1991). "Chemical and elemental analysis of humans in vivo using improved body composition models".The American Journal of Physiology.261(2 Pt 1): E190-8.doi:10.1152/ajpendo.1991.261.2.E190.PMID1872381.
- ^"Electrolyte Balance".Anatomy and Physiology.OpenStax. Archived fromthe originalon 2 June 2020.Retrieved23 June2020.
- ^Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000)."The Action Potential and Conduction of Electric Impulses".Molecular Cell Biology(4th ed.).Archivedfrom the original on 30 May 2020.Retrieved23 June2020– via NCBI.
- ^Dulhunty AF (September 2006). "Excitation-contraction coupling from the 1950s into the new millennium".Clinical and Experimental Pharmacology & Physiology.33(9): 763–72.doi:10.1111/j.1440-1681.2006.04441.x.PMID16922804.S2CID37462321.
- ^Torres-Romero JC, Alvarez-Sánchez ME, Fernández-Martín K, Alvarez-Sánchez LC, Arana-Argáez V, Ramírez-Camacho M, Lara-Riegos J (2018). "Zinc Efflux in Trichomonas vaginalis: In Silico Identification and Expression Analysis of CDF-Like Genes". In Olivares-Quiroz L, Resendis-Antonio O (eds.).Quantitative Models for Microscopic to Macroscopic Biological Macromolecules and Tissues.Cham: Springer International Publishing. pp. 149–168.doi:10.1007/978-3-319-73975-5_8.ISBN978-3-319-73975-5.
- ^Cousins RJ, Liuzzi JP, Lichten LA (August 2006)."Mammalian zinc transport, trafficking, and signals".The Journal of Biological Chemistry.281(34): 24085–9.doi:10.1074/jbc.R600011200.PMID16793761.Archivedfrom the original on 25 June 2020.Retrieved24 June2020.
- ^Dunn LL, Suryo Rahmanto Y, Richardson DR (February 2007). "Iron uptake and metabolism in the new millennium".Trends in Cell Biology.17(2): 93–100.doi:10.1016/j.tcb.2006.12.003.PMID17194590.
- ^abAlberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "How Cells Obtain Energy from Food".Molecular Biology of the Cell(4th ed.).Archivedfrom the original on 5 July 2021.Retrieved25 June2020– via NCBI.
- ^Raven J (3 September 2009)."Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments".Aquatic Microbial Ecology.56:177–192.doi:10.3354/ame01315.ISSN0948-3055.Archivedfrom the original on 25 June 2020.Retrieved25 June2020.
- ^abNelson N, Ben-Shem A (December 2004). "The complex architecture of oxygenic photosynthesis".Nature Reviews. Molecular Cell Biology.5(12): 971–82.doi:10.1038/nrm1525.PMID15573135.S2CID5686066.
- ^Madigan MT, Martinko JM (2006).Brock Mikrobiologie(11., überarb. Aufl ed.). München: Pearson Studium. pp. 604, 621.ISBN3-8273-7187-2.OCLC162303067.
- ^Demirel Y (2016).Energy: production, conversion, storage, conservation, and coupling(Second ed.). Lincoln: Springer. p. 431.ISBN978-3-319-29650-0.OCLC945435943.
- ^Häse CC, Finkelstein RA (December 1993)."Bacterial extracellular zinc-containing metalloproteases".Microbiological Reviews.57(4): 823–37.doi:10.1128/MMBR.57.4.823-837.1993.PMC372940.PMID8302217.
- ^Gupta R, Gupta N, Rathi P (June 2004). "Bacterial lipases: an overview of production, purification and biochemical properties".Applied Microbiology and Biotechnology.64(6): 763–81.doi:10.1007/s00253-004-1568-8.PMID14966663.S2CID206934353.
- ^Hoyle T (1997). "The digestive system: linking theory and practice".British Journal of Nursing.6(22): 1285–91.doi:10.12968/bjon.1997.6.22.1285.PMID9470654.
- ^Souba WW, Pacitti AJ (1992). "How amino acids get into cells: mechanisms, models, menus, and mediators".Journal of Parenteral and Enteral Nutrition.16(6): 569–78.doi:10.1177/0148607192016006569.PMID1494216.
- ^Barrett MP, Walmsley AR, Gould GW (August 1999). "Structure and function of facilitative sugar transporters".Current Opinion in Cell Biology.11(4): 496–502.doi:10.1016/S0955-0674(99)80072-6.PMID10449337.
- ^Bell GI, Burant CF, Takeda J, Gould GW (September 1993)."Structure and function of mammalian facilitative sugar transporters".The Journal of Biological Chemistry.268(26): 19161–4.doi:10.1016/S0021-9258(19)36489-0.PMID8366068.
- ^abBouché C, Serdy S, Kahn CR, Goldfine AB (October 2004)."The cellular fate of glucose and its relevance in type 2 diabetes".Endocrine Reviews.25(5): 807–30.doi:10.1210/er.2003-0026.PMID15466941.
- ^Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, et al. (18 December 2014)."Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question".Oncoscience.1(12): 777–802.doi:10.18632/oncoscience.109.PMC4303887.PMID25621294.
- ^Kruger, Nicholas J; von Schaewen, Antje (2003). "The oxidative pentose phosphate pathway: structure and organisation".Current Opinion in Plant Biology.6(3): 236–246.Bibcode:2003COPB....6..236K.doi:10.1016/S1369-5266(03)00039-6.PMID12753973.
- ^Wipperman MF, Sampson NS, Thomas ST (2014)."Pathogen roid rage: cholesterol utilization by Mycobacterium tuberculosis".Critical Reviews in Biochemistry and Molecular Biology.49(4): 269–93.doi:10.3109/10409238.2014.895700.PMC4255906.PMID24611808.
- ^Sakami W, Harrington H (1963). "Amino Acid Metabolism".Annual Review of Biochemistry.32:355–98.doi:10.1146/annurev.bi.32.070163.002035.PMID14144484.
- ^Brosnan JT (April 2000)."Glutamate, at the interface between amino acid and carbohydrate metabolism".The Journal of Nutrition.130(4S Suppl): 988S–90S.doi:10.1093/jn/130.4.988S.PMID10736367.
- ^Young VR, Ajami AM (September 2001)."Glutamine: the emperor or his clothes?".The Journal of Nutrition.131(9 Suppl): 2449S–59S, discussion 2486S–7S.doi:10.1093/jn/131.9.2449S.PMID11533293.
- ^Hosler JP, Ferguson-Miller S, Mills DA (2006)."Energy transduction: proton transfer through the respiratory complexes".Annual Review of Biochemistry.75:165–87.doi:10.1146/annurev.biochem.75.062003.101730.PMC2659341.PMID16756489.
- ^Schultz BE, Chan SI (2001)."Structures and proton-pumping strategies of mitochondrial respiratory enzymes"(PDF).Annual Review of Biophysics and Biomolecular Structure.30:23–65.doi:10.1146/annurev.biophys.30.1.23.PMID11340051.Archived(PDF)from the original on 22 January 2020.Retrieved11 November2019.
- ^Capaldi RA, Aggeler R (March 2002). "Mechanism of the F(1)F(0)-type ATP synthase, a biological rotary motor".Trends in Biochemical Sciences.27(3): 154–60.doi:10.1016/S0968-0004(01)02051-5.PMID11893513.
- ^Friedrich B, Schwartz E (1993). "Molecular biology of hydrogen utilization in aerobic chemolithotrophs".Annual Review of Microbiology.47:351–83.doi:10.1146/annurev.mi.47.100193.002031.PMID8257102.
- ^Weber KA, Achenbach LA, Coates JD (October 2006)."Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction".Nature Reviews. Microbiology.4(10): 752–64.doi:10.1038/nrmicro1490.PMID16980937.S2CID8528196.Archivedfrom the original on 2 May 2019.Retrieved6 October2019.
- ^Jetten MS, Strous M, van de Pas-Schoonen KT, Schalk J, van Dongen UG, van de Graaf AA, et al. (December 1998)."The anaerobic oxidation of ammonium".FEMS Microbiology Reviews.22(5): 421–37.doi:10.1111/j.1574-6976.1998.tb00379.x.PMID9990725.
- ^Simon J (August 2002)."Enzymology and bioenergetics of respiratory nitrite ammonification".FEMS Microbiology Reviews.26(3): 285–309.doi:10.1111/j.1574-6976.2002.tb00616.x.PMID12165429.
- ^Conrad R (December 1996)."Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO)".Microbiological Reviews.60(4): 609–40.doi:10.1128/MMBR.60.4.609-640.1996.PMC239458.PMID8987358.
- ^Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (July 2005)."Microbial co-operation in the rhizosphere".Journal of Experimental Botany.56(417): 1761–78.doi:10.1093/jxb/eri197.PMID15911555.
- ^van der Meer MT, Schouten S, Bateson MM, Nübel U, Wieland A, Kühl M, et al. (July 2005)."Diel variations in carbon metabolism by green nonsulfur-like bacteria in alkaline siliceous hot spring microbial mats from Yellowstone National Park".Applied and Environmental Microbiology.71(7): 3978–86.Bibcode:2005ApEnM..71.3978V.doi:10.1128/AEM.71.7.3978-3986.2005.PMC1168979.PMID16000812.
- ^Tichi MA, Tabita FR (November 2001)."Interactive control of Rhodobacter capsulatus redox-balancing systems during phototrophic metabolism".Journal of Bacteriology.183(21): 6344–54.doi:10.1128/JB.183.21.6344-6354.2001.PMC100130.PMID11591679.
- ^Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Energy Conversion: Mitochondria and Chloroplasts".Molecular Biology of the Cell(4th ed.).Archivedfrom the original on 15 December 2020.Retrieved3 July2020.
- ^Allen JP, Williams JC (October 1998). "Photosynthetic reaction centers".FEBS Letters.438(1–2): 5–9.Bibcode:1998FEBSL.438....5A.doi:10.1016/S0014-5793(98)01245-9.PMID9821949.S2CID21596537.
- ^Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (June 2004). "Cyclic electron flow around photosystem I is essential for photosynthesis".Nature.429(6991): 579–82.Bibcode:2004Natur.429..579M.doi:10.1038/nature02598.PMID15175756.S2CID4421776.
- ^abMandal A (26 November 2009)."What is Anabolism?".News-Medical.net.Archivedfrom the original on 5 July 2020.Retrieved4 July2020.
- ^Miziorko HM, Lorimer GH (1983). "Ribulose-1,5-bisphosphate carboxylase-oxygenase".Annual Review of Biochemistry.52:507–35.doi:10.1146/annurev.bi.52.070183.002451.PMID6351728.
- ^Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (April 2002)."Crassulacean acid metabolism: plastic, fantastic".Journal of Experimental Botany.53(369): 569–80.doi:10.1093/jexbot/53.369.569.PMID11886877.
- ^Hügler M, Wirsen CO, Fuchs G, Taylor CD, Sievert SM (May 2005)."Evidence for autotrophic CO2 fixation via the reductive tricarboxylic acid cycle by members of the epsilon subdivision of proteobacteria".Journal of Bacteriology.187(9): 3020–7.doi:10.1128/JB.187.9.3020-3027.2005.PMC1082812.PMID15838028.
- ^Strauss G, Fuchs G (August 1993)."Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle".European Journal of Biochemistry.215(3): 633–43.doi:10.1111/j.1432-1033.1993.tb18074.x.PMID8354269.
- ^Wood HG (February 1991)."Life with CO or CO2 and H2 as a source of carbon and energy".FASEB Journal.5(2): 156–63.doi:10.1096/fasebj.5.2.1900793.PMID1900793.S2CID45967404.
- ^Shively JM, van Keulen G, Meijer WG (1998). "Something from almost nothing: carbon dioxide fixation in chemoautotrophs".Annual Review of Microbiology.52:191–230.doi:10.1146/annurev.micro.52.1.191.PMID9891798.
- ^Boiteux A, Hess B (June 1981)."Design of glycolysis".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.293(1063): 5–22.Bibcode:1981RSPTB.293....5B.doi:10.1098/rstb.1981.0056.PMID6115423.
- ^Pilkis SJ, el-Maghrabi MR, Claus TH (June 1990). "Fructose-2,6-bisphosphate in control of hepatic gluconeogenesis. From metabolites to molecular genetics".Diabetes Care.13(6): 582–99.doi:10.2337/diacare.13.6.582.PMID2162755.S2CID44741368.
- ^abEnsign SA (July 2006)."Revisiting the glyoxylate cycle: alternate pathways for microbial acetate assimilation".Molecular Microbiology.61(2): 274–6.doi:10.1111/j.1365-2958.2006.05247.x.PMID16856935.S2CID39986630.
- ^Finn PF, Dice JF (2006). "Proteolytic and lipolytic responses to starvation".Nutrition.22(7–8): 830–44.doi:10.1016/j.nut.2006.04.008.PMID16815497.
- ^abKornberg HL, Krebs HA (May 1957). "Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle".Nature.179(4568): 988–91.Bibcode:1957Natur.179..988K.doi:10.1038/179988a0.PMID13430766.S2CID40858130.
- ^Evans RD, Heather LC (June 2016)."Metabolic pathways and abnormalities".Surgery (Oxford).34(6): 266–272.doi:10.1016/j.mpsur.2016.03.010.ISSN0263-9319.S2CID87884121.Archivedfrom the original on 31 October 2020.Retrieved28 August2020.
- ^Freeze HH, Hart GW, Schnaar RL (2015). "Glycosylation Precursors". In Varki A, Cummings RD, Esko JD, Stanley P (eds.).Essentials of Glycobiology(3rd ed.). Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press.doi:10.1101/glycobiology.3e.005(inactive 11 September 2024).PMID28876856.Archivedfrom the original on 24 February 2022.Retrieved8 July2020.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^Opdenakker G, Rudd PM, Ponting CP, Dwek RA (November 1993)."Concepts and principles of glycobiology".FASEB Journal.7(14): 1330–7.doi:10.1096/fasebj.7.14.8224606.PMID8224606.S2CID10388991.
- ^McConville MJ, Menon AK (2000)."Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids (review)".Molecular Membrane Biology.17(1): 1–16.doi:10.1080/096876800294443.PMID10824734.
- ^Chirala SS, Wakil SJ (November 2004). "Structure and function of animal fatty acid synthase".Lipids.39(11): 1045–53.doi:10.1007/s11745-004-1329-9.PMID15726818.S2CID4043407.
- ^White SW, Zheng J, Zhang YM (2005). "The structural biology of type II fatty acid biosynthesis".Annual Review of Biochemistry.74:791–831.doi:10.1146/annurev.biochem.74.082803.133524.PMID15952903.
- ^Ohlrogge JB, Jaworski JG (June 1997). "Regulation of Fatty Acid Synthesis".Annual Review of Plant Physiology and Plant Molecular Biology.48:109–136.doi:10.1146/annurev.arplant.48.1.109.PMID15012259.S2CID46348092.
- ^Dubey VS, Bhalla R, Luthra R (September 2003)."An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants"(PDF).Journal of Biosciences.28(5): 637–46.doi:10.1007/BF02703339.PMID14517367.S2CID27523830.Archived fromthe original(PDF)on 15 April 2007.
- ^abKuzuyama T, Seto H (April 2003). "Diversity of the biosynthesis of the isoprene units".Natural Product Reports.20(2): 171–83.doi:10.1039/b109860h.PMID12735695.
- ^Grochowski LL, Xu H, White RH (May 2006)."Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate".Journal of Bacteriology.188(9): 3192–8.doi:10.1128/JB.188.9.3192-3198.2006.PMC1447442.PMID16621811.
- ^Lichtenthaler HK (June 1999). "The 1-Deoxy-D-Xylulose-5-Phosphate Pathway of Isoprenoid Biosynthesis in Plants".Annual Review of Plant Physiology and Plant Molecular Biology.50:47–65.doi:10.1146/annurev.arplant.50.1.47.PMID15012203.
- ^abSchroepfer GJ (1981). "Sterol biosynthesis".Annual Review of Biochemistry.50:585–621.doi:10.1146/annurev.bi.50.070181.003101.PMID7023367.
- ^Lees ND, Skaggs B, Kirsch DR, Bard M (March 1995). "Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae--a review".Lipids.30(3): 221–6.doi:10.1007/BF02537824.PMID7791529.S2CID4019443.
- ^Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li BC, Herrmann R (November 1996)."Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae".Nucleic Acids Research.24(22): 4420–49.doi:10.1093/nar/24.22.4420.PMC146264.PMID8948633.
- ^Guyton AC, Hall JE (2006).Textbook of Medical Physiology.Philadelphia: Elsevier. pp.855–6.ISBN978-0-7216-0240-0.
- ^Ibba M, Söll D (May 2001)."The renaissance of aminoacyl-tRNA synthesis".EMBO Reports.2(5): 382–7.doi:10.1093/embo-reports/kve095.PMC1083889.PMID11375928.Archived fromthe originalon 1 May 2011.
- ^Lengyel P, Söll D (June 1969)."Mechanism of protein biosynthesis".Bacteriological Reviews.33(2): 264–301.doi:10.1128/MMBR.33.2.264-301.1969.PMC378322.PMID4896351.
- ^abRudolph FB (January 1994)."The biochemistry and physiology of nucleotides".The Journal of Nutrition.124(1 Suppl): 124S–127S.doi:10.1093/jn/124.suppl_1.124S.PMID8283301.Zrenner R, Stitt M, Sonnewald U, Boldt R (2006). "Pyrimidine and purine biosynthesis and degradation in plants".Annual Review of Plant Biology.57:805–36.doi:10.1146/annurev.arplant.57.032905.105421.PMID16669783.
- ^Stasolla C, Katahira R, Thorpe TA, Ashihara H (November 2003). "Purine and pyrimidine nucleotide metabolism in higher plants".Journal of Plant Physiology.160(11): 1271–95.Bibcode:2003JPPhy.160.1271S.doi:10.1078/0176-1617-01169.PMID14658380.
- ^Davies O, Mendes P, Smallbone K, Malys N (April 2012)."Characterisation of multiple substrate-specific (d)ITP/(d)XTPase and modelling of deaminated purine nucleotide metabolism"(PDF).BMB Reports.45(4): 259–64.doi:10.5483/BMBRep.2012.45.4.259.PMID22531138.Archived(PDF)from the original on 24 October 2020.Retrieved18 September2019.
- ^Smith JL (December 1995). "Enzymes of nucleotide synthesis".Current Opinion in Structural Biology.5(6): 752–7.doi:10.1016/0959-440X(95)80007-7.PMID8749362.
- ^Testa B, Krämer SD (October 2006). "The biochemistry of drug metabolism--an introduction: part 1. Principles and overview".Chemistry & Biodiversity.3(10): 1053–101.doi:10.1002/cbdv.200690111.PMID17193224.S2CID28872968.
- ^Danielson PB (December 2002). "The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans".Current Drug Metabolism.3(6): 561–97.doi:10.2174/1389200023337054.PMID12369887.
- ^King CD, Rios GR, Green MD, Tephly TR (September 2000). "UDP-glucuronosyltransferases".Current Drug Metabolism.1(2): 143–61.doi:10.2174/1389200003339171.PMID11465080.
- ^Sheehan D, Meade G, Foley VM, Dowd CA (November 2001)."Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily".The Biochemical Journal.360(Pt 1): 1–16.doi:10.1042/0264-6021:3600001.PMC1222196.PMID11695986.
- ^Galvão TC, Mohn WW, de Lorenzo V (October 2005). "Exploring the microbial biodegradation and biotransformation gene pool".Trends in Biotechnology.23(10): 497–506.doi:10.1016/j.tibtech.2005.08.002.PMID16125262.
- ^Janssen DB, Dinkla IJ, Poelarends GJ, Terpstra P (December 2005)."Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities"(PDF).Environmental Microbiology.7(12): 1868–82.Bibcode:2005EnvMi...7.1868J.doi:10.1111/j.1462-2920.2005.00966.x.PMID16309386.Archived(PDF)from the original on 11 November 2019.Retrieved11 November2019.
- ^Davies KJ (1995). "Oxidative stress: the paradox of aerobic life".Biochemical Society Symposium.61:1–31.doi:10.1042/bss0610001.PMID8660387.
- ^Tu BP, Weissman JS (February 2004)."Oxidative protein folding in eukaryotes: mechanisms and consequences".The Journal of Cell Biology.164(3): 341–6.doi:10.1083/jcb.200311055.PMC2172237.PMID14757749.
- ^Sies H (March 1997)."Oxidative stress: oxidants and antioxidants".Experimental Physiology.82(2): 291–5.doi:10.1113/expphysiol.1997.sp004024.PMID9129943.S2CID20240552.
- ^Vertuani S, Angusti A, Manfredini S (2004). "The antioxidants and pro-antioxidants network: an overview".Current Pharmaceutical Design.10(14): 1677–94.doi:10.2174/1381612043384655.PMID15134565.S2CID43713549.
- ^von Stockar U, Liu J (August 1999)."Does microbial life always feed on negative entropy? Thermodynamic analysis of microbial growth".Biochimica et Biophysica Acta (BBA) - Bioenergetics.1412(3): 191–211.doi:10.1016/S0005-2728(99)00065-1.PMID10482783.
- ^Demirel Y, Sandler SI (June 2002)."Thermodynamics and bioenergetics".Biophysical Chemistry.97(2–3): 87–111.doi:10.1016/S0301-4622(02)00069-8.PMID12050002.S2CID3754065.Archivedfrom the original on 4 August 2020.Retrieved22 September2019.
- ^Albert R (November 2005). "Scale-free networks in cell biology".Journal of Cell Science.118(Pt 21): 4947–57.arXiv:q-bio/0510054.Bibcode:2005q.bio....10054A.doi:10.1242/jcs.02714.PMID16254242.S2CID3001195.
- ^Brand MD (January 1997)."Regulation analysis of energy metabolism".The Journal of Experimental Biology.200(Pt 2): 193–202.doi:10.1242/jeb.200.2.193.PMID9050227.Archivedfrom the original on 29 March 2007.Retrieved12 March2007.
- ^Soyer OS, Salathé M, Bonhoeffer S (January 2006). "Signal transduction networks: topology, response and biochemical processes".Journal of Theoretical Biology.238(2): 416–25.Bibcode:2006JThBi.238..416S.doi:10.1016/j.jtbi.2005.05.030.PMID16045939.
- ^abSalter M, Knowles RG, Pogson CI (1994). "Metabolic control".Essays in Biochemistry.28:1–12.PMID7925313.
- ^Westerhoff HV, Groen AK, Wanders RJ (January 1984). "Modern theories of metabolic control and their applications (review)".Bioscience Reports.4(1): 1–22.doi:10.1007/BF01120819.PMID6365197.S2CID27791605.
- ^Fell DA, Thomas S (October 1995)."Physiological control of metabolic flux: the requirement for multisite modulation".The Biochemical Journal.311(Pt 1): 35–9.doi:10.1042/bj3110035.PMC1136115.PMID7575476.
- ^Hendrickson WA (November 2005). "Transduction of biochemical signals across cell membranes".Quarterly Reviews of Biophysics.38(4): 321–30.doi:10.1017/S0033583506004136.PMID16600054.S2CID39154236.
- ^Cohen P (December 2000). "The regulation of protein function by multisite phosphorylation--a 25 year update".Trends in Biochemical Sciences.25(12): 596–601.doi:10.1016/S0968-0004(00)01712-6.PMID11116185.
- ^Lienhard GE, Slot JW, James DE, Mueckler MM (January 1992). "How cells absorb glucose".Scientific American.266(1): 86–91.Bibcode:1992SciAm.266a..86L.doi:10.1038/scientificamerican0192-86.PMID1734513.
- ^Roach PJ (March 2002). "Glycogen and its metabolism".Current Molecular Medicine.2(2): 101–20.doi:10.2174/1566524024605761.PMID11949930.
- ^Newgard CB, Brady MJ, O'Doherty RM, Saltiel AR (December 2000)."Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1"(PDF).Diabetes.49(12): 1967–77.doi:10.2337/diabetes.49.12.1967.PMID11117996.Archived(PDF)from the original on 19 June 2007.Retrieved25 March2007.
- ^Romano AH, Conway T (1996)."Evolution of carbohydrate metabolic pathways".Research in Microbiology.147(6–7): 448–55.doi:10.1016/0923-2508(96)83998-2.PMID9084754.
- ^Koch A (1998).How Did Bacteria Come to Be?.Advances in Microbial Physiology. Vol. 40. pp. 353–99.doi:10.1016/S0065-2911(08)60135-6.ISBN978-0-12-027740-7.PMID9889982.
{{cite book}}:|journal=ignored (help) - ^Ouzounis C, Kyrpides N (July 1996)."The emergence of major cellular processes in evolution".FEBS Letters.390(2): 119–23.Bibcode:1996FEBSL.390..119O.doi:10.1016/0014-5793(96)00631-X.PMID8706840.S2CID39128865.
- ^Caetano-Anollés G, Kim HS, Mittenthal JE (May 2007)."The origin of modern metabolic networks inferred from phylogenomic analysis of protein architecture".Proceedings of the National Academy of Sciences of the United States of America.104(22): 9358–63.Bibcode:2007PNAS..104.9358C.doi:10.1073/pnas.0701214104.PMC1890499.PMID17517598.
- ^Schmidt S, Sunyaev S, Bork P, Dandekar T (June 2003). "Metabolites: a helping hand for pathway evolution?".Trends in Biochemical Sciences.28(6): 336–41.doi:10.1016/S0968-0004(03)00114-2.PMID12826406.
- ^Light S, Kraulis P (February 2004)."Network analysis of metabolic enzyme evolution in Escherichia coli".BMC Bioinformatics.5:15.doi:10.1186/1471-2105-5-15.PMC394313.PMID15113413.Alves R, Chaleil RA, Sternberg MJ (July 2002). "Evolution of enzymes in metabolism: a network perspective".Journal of Molecular Biology.320(4): 751–70.doi:10.1016/S0022-2836(02)00546-6.PMID12095253.
- ^Kim HS, Mittenthal JE, Caetano-Anollés G (July 2006)."MANET: tracing evolution of protein architecture in metabolic networks".BMC Bioinformatics.7:351.doi:10.1186/1471-2105-7-351.PMC1559654.PMID16854231.
- ^Teichmann SA, Rison SC, Thornton JM, Riley M, Gough J, Chothia C (December 2001). "Small-molecule metabolism: an enzyme mosaic".Trends in Biotechnology.19(12): 482–6.doi:10.1016/S0167-7799(01)01813-3.PMID11711174.
- ^Spirin V, Gelfand MS, Mironov AA, Mirny LA (June 2006)."A metabolic network in the evolutionary context: multiscale structure and modularity".Proceedings of the National Academy of Sciences of the United States of America.103(23): 8774–9.Bibcode:2006PNAS..103.8774S.doi:10.1073/pnas.0510258103.PMC1482654.PMID16731630.
- ^Lawrence JG (December 2005). "Common themes in the genome strategies of pathogens".Current Opinion in Genetics & Development.15(6): 584–8.doi:10.1016/j.gde.2005.09.007.PMID16188434.Wernegreen JJ (December 2005). "For better or worse: genomic consequences of intracellular mutualism and parasitism".Current Opinion in Genetics & Development.15(6): 572–83.doi:10.1016/j.gde.2005.09.013.PMID16230003.
- ^Pál C, Papp B, Lercher MJ, Csermely P, Oliver SG, Hurst LD (March 2006). "Chance and necessity in the evolution of minimal metabolic networks".Nature.440(7084): 667–70.Bibcode:2006Natur.440..667P.doi:10.1038/nature04568.PMID16572170.S2CID4424895.
- ^Rennie MJ (November 1999)."An introduction to the use of tracers in nutrition and metabolism".The Proceedings of the Nutrition Society.58(4): 935–44.doi:10.1017/S002966519900124X.PMID10817161.
- ^Phair RD (December 1997)."Development of kinetic models in the nonlinear world of molecular cell biology".Metabolism.46(12): 1489–95.doi:10.1016/S0026-0495(97)90154-2.PMID9439549.
- ^Sterck L, Rombauts S, Vandepoele K, Rouzé P, Van de Peer Y (April 2007). "How many genes are there in plants (... and why are they there)?".Current Opinion in Plant Biology.10(2): 199–203.doi:10.1016/j.pbi.2007.01.004.PMID17289424.
- ^Borodina I, Nielsen J (June 2005). "From genomes to in silico cells via metabolic networks".Current Opinion in Biotechnology.16(3): 350–5.doi:10.1016/j.copbio.2005.04.008.PMID15961036.
- ^Gianchandani EP, Brautigan DL, Papin JA (May 2006). "Systems analyses characterize integrated functions of biochemical networks".Trends in Biochemical Sciences.31(5): 284–91.doi:10.1016/j.tibs.2006.03.007.PMID16616498.
- ^Duarte NC, Becker SA, Jamshidi N, Thiele I, Mo ML, Vo TD, et al. (February 2007)."Global reconstruction of the human metabolic network based on genomic and bibliomic data".Proceedings of the National Academy of Sciences of the United States of America.104(6): 1777–82.Bibcode:2007PNAS..104.1777D.doi:10.1073/pnas.0610772104.PMC1794290.PMID17267599.
- ^Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabási AL (May 2007)."The human disease network".Proceedings of the National Academy of Sciences of the United States of America.104(21): 8685–90.Bibcode:2007PNAS..104.8685G.doi:10.1073/pnas.0701361104.PMC1885563.PMID17502601.
- ^Lee DS, Park J, Kay KA, Christakis NA, Oltvai ZN, Barabási AL (July 2008)."The implications of human metabolic network topology for disease comorbidity".Proceedings of the National Academy of Sciences of the United States of America.105(29): 9880–5.Bibcode:2008PNAS..105.9880L.doi:10.1073/pnas.0802208105.PMC2481357.PMID18599447.
- ^Csete M, Doyle J (September 2004). "Bow ties, metabolism and disease".Trends in Biotechnology.22(9): 446–50.doi:10.1016/j.tibtech.2004.07.007.PMID15331224.
- ^Ma HW, Zeng AP (July 2003). "The connectivity structure, giant strong component and centrality of metabolic networks".Bioinformatics.19(11): 1423–30.CiteSeerX10.1.1.605.8964.doi:10.1093/bioinformatics/btg177.PMID12874056.
- ^Zhao J, Yu H, Luo JH, Cao ZW, Li YX (August 2006)."Hierarchical modularity of nested bow-ties in metabolic networks".BMC Bioinformatics.7:386.arXiv:q-bio/0605003.Bibcode:2006q.bio.....5003Z.doi:10.1186/1471-2105-7-386.PMC1560398.PMID16916470.
- ^"Macromolecules: Nutrients, Metabolism, and Digestive Processes | Virtual High School - KeepNotes".keepnotes.com.Archived fromthe originalon 29 December 2023.Retrieved29 December2023.
- ^Thykaer J, Nielsen J (January 2003). "Metabolic engineering of beta-lactam production".Metabolic Engineering.5(1): 56–69.doi:10.1016/S1096-7176(03)00003-X.PMID12749845.
- ^González-Pajuelo M, Meynial-Salles I, Mendes F, Andrade JC, Vasconcelos I, Soucaille P (2005). "Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol".Metabolic Engineering.7(5–6): 329–36.doi:10.1016/j.ymben.2005.06.001.hdl:10400.14/3388.PMID16095939.
- ^Krämer M, Bongaerts J, Bovenberg R, Kremer S, Müller U, Orf S, et al. (October 2003). "Metabolic engineering for microbial production of shikimic acid".Metabolic Engineering.5(4): 277–83.doi:10.1016/j.ymben.2003.09.001.PMID14642355.
- ^Koffas M, Roberge C, Lee K, Stephanopoulos G (1999). "Metabolic engineering".Annual Review of Biomedical Engineering.1:535–57.doi:10.1146/annurev.bioeng.1.1.535.PMID11701499.S2CID11814282.
- ^"metabolism | Origin and meaning of metabolism by Online Etymology Dictionary".www.etymonline.com.Archivedfrom the original on 21 September 2017.Retrieved23 July2020.
- ^Leroi, Armand Marie(2014).The Lagoon: How Aristotle Invented Science.Bloomsbury. pp. 400–401.ISBN978-1-4088-3622-4.
- ^Al-Roubi AS (1982).Ibn Al-Nafis as a philosopher.Symposium on Ibn al-Nafis, Second International Conference on Islamic Medicine. Kuwait: Islamic Medical Organization.
- ^Eknoyan G (1999). "Santorio Sanctorius (1561-1636) - founding father of metabolic balance studies".American Journal of Nephrology.19(2): 226–33.doi:10.1159/000013455.PMID10213823.S2CID32900603.
- ^Williams HA (1904).Modern Development of the Chemical and Biological Sciences.A History of Science: in Five Volumes. Vol. IV. New York: Harper and Brothers. pp. 184–185.Retrieved26 March2007.
- ^Manchester KL (December 1995). "Louis Pasteur (1822-1895)--chance and the prepared mind".Trends in Biotechnology.13(12): 511–5.doi:10.1016/S0167-7799(00)89014-9.PMID8595136.
- ^Kinne-Saffran E, Kinne RK (1999). "Vitalism and synthesis of urea. From Friedrich Wöhler to Hans A. Krebs".American Journal of Nephrology.19(2): 290–4.doi:10.1159/000013463.PMID10213830.S2CID71727190.
- ^Eduard Buchner's 1907Nobel lectureArchived8 July 2017 at theWayback Machineathttp://nobelprize.orgArchived5 April 2006 at theWayback MachineAccessed 20 March 2007
- ^Kornberg H (December 2000). "Krebs and his trinity of cycles".Nature Reviews. Molecular Cell Biology.1(3): 225–8.doi:10.1038/35043073.PMID11252898.S2CID28092593.
- ^Krebs HA, Henseleit K (1932). "Untersuchungen über die Harnstoffbildung im tierkorper".Z. Physiol. Chem.210(1–2): 33–66.doi:10.1515/bchm2.1932.210.1-2.33.
- ^Krebs HA, Johnson WA (April 1937)."Metabolism of ketonic acids in animal tissues".The Biochemical Journal.31(4): 645–60.doi:10.1042/bj0310645.PMC1266984.PMID16746382.
Further reading
[edit]Introductory
- Rose S, Mileusnic R (1999).The Chemistry of Life.Penguin Press Science.ISBN0-14-027273-9.
- Schneider EC, Sagan D (2005).Into the Cool: Energy Flow, Thermodynamics, and Life.University of Chicago Press.ISBN0-226-73936-8.
- Lane N (2004).Oxygen: The Molecule that Made the World.USA: Oxford University Press.ISBN0-19-860783-0.
Advanced
- Price N, Stevens L (1999).Fundamentals of Enzymology: Cell and Molecular Biology of Catalytic Proteins.Oxford University Press.ISBN0-19-850229-X.
- Berg J, Tymoczko J, Stryer L (2002).Biochemistry.W. H. Freeman and Company.ISBN0-7167-4955-6.
- Cox M, Nelson DL (2004).Lehninger Principles of Biochemistry.Palgrave Macmillan.ISBN0-7167-4339-6.
- Brock TD,Madigan MR, Martinko J, Parker J (2002).Brock's Biology of Microorganisms.Benjamin Cummings.ISBN0-13-066271-2.
- Da Silva JJ, Williams RJ (1991).The Biological Chemistry of the Elements: The Inorganic Chemistry of Life.Clarendon Press.ISBN0-19-855598-9.
- Nicholls DG, Ferguson SJ (2002).Bioenergetics.Academic Press Inc.ISBN0-12-518121-3.
- Wood HG (February 1991)."Life with CO or CO2 and H2 as a source of carbon and energy".FASEB Journal.5(2): 156–63.doi:10.1096/fasebj.5.2.1900793.PMID1900793.S2CID45967404.
External links
[edit]General information
- The Biochemistry of Metabolism(archived 8 March 2005)
- Sparknotes SAT biochemistryOverview of biochemistry. School level.
- MIT Biology HypertextbookArchived19 May 2016 at the Portuguese Web Archive Undergraduate-level guide to molecular biology.
Human metabolism
- Topics in Medical BiochemistryGuide to human metabolic pathways. School level.
- THE Medical Biochemistry PageComprehensive resource on human metabolism.
Databases
- Flow Chart of Metabolic PathwaysatExPASy
- IUBMB-Nicholson Metabolic Pathways Chart
- SuperCYP: Database for Drug-Cytochrome-MetabolismArchived3 November 2011 at theWayback Machine
Metabolic pathways
- Metabolism reference PathwayArchived23 February 2009 at theWayback Machine
- The Nitrogen cycle and Nitrogen fixationat theWayback Machine(archive index)
- ^Beebe, Jane A.; Frey, Perry A. (1 October 1998)."Galactose Mutarotase: Purification, Characterization, and Investigations of Two Important Histidine Residues".Biochemistry.37(42): 14989–14997.doi:10.1021/bi9816047.ISSN0006-2960.