Metalloprotein

Metalloproteinis a generic term for aproteinthat contains a metal ioncofactor.[1][2]A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-bindingprotein domains[3]although there may be up to 3000 human zinc metalloproteins.[4]
Abundance
[edit]It is estimated that approximately half of allproteinscontain ametal.[5]In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions.[6]Thus, metalloproteins have many different functions incells,such as storage and transport of proteins,enzymesandsignal transductionproteins, or infectious diseases.[7]The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals.[8]
Most metals in thehumanbodyare bound to proteins. For instance, the relatively high concentration of iron in the human body is mostly due to the iron inhemoglobin.
| Liver | Kidney | Lung | Heart | Brain | Muscle | |
|---|---|---|---|---|---|---|
| Mn (manganese) | 138 | 79 | 29 | 27 | 22 | <4-40 |
| Fe (iron) | 16,769 | 7,168 | 24,967 | 5,530 | 4,100 | 3,500 |
| Co (cobalt) | <2-13 | <2 | <2-8 | --- | <2 | 150 (?) |
| Ni (nickel) | <5 | <5-12 | <5 | <5 | <5 | <15 |
| Cu (copper) | 882 | 379 | 220 | 350 | 401 | 85-305 |
| Zn (zinc) | 5,543 | 5,018 | 1,470 | 2,772 | 915 | 4,688 |
Coordination chemistry principles
[edit]In metalloproteins, metal ions are usually coordinated bynitrogen,oxygenorsulfurcenters belonging toamino acidresidues of the protein. These donor groups are often provided by side-chains on the amino acid residues. Especially important are theimidazolesubstituent inhistidineresidues,thiolatesubstituents incysteineresidues, andcarboxylategroups provided byaspartate.Given the diversity of the metalloproteome,virtually all amino acid residues have been shown to bind metal centers. The peptide backbone also provides donor groups; these include deprotonatedamidesand the amidecarbonyloxygen centers. Lead(II) binding in natural and artificial proteins has been reviewed.[10]
In addition to donor groups that are provided by amino acid residues, many organiccofactorsfunction as ligands. Perhaps most famous are the tetradentate N4macrocyclicligandsincorporated into thehemeprotein. Inorganic ligands such as sulfide and oxide are also common.
Storage and transport metalloproteins
[edit]These are the second stage product of protein hydrolysis obtained by treatment with slightly stronger acids and alkalies.
Oxygen carriers
[edit]Hemoglobin,which is the principal oxygen-carrier in humans, has four subunits in which theiron(II) ion is coordinated by the planarmacrocyclicligandprotoporphyrin IX(PIX) and theimidazolenitrogen atom of ahistidineresidue. The sixth coordination site contains awatermolecule or adioxygenmolecule. By contrast the proteinmyoglobin,found inmuscle cells,has only one such unit. The active site is located in ahydrophobicpocket. This is important as without it the iron(II) would be irreversiblyoxidizedto iron(III). Theequilibrium constantfor the formation of HbO2is such that oxygen is taken up or released depending on thepartial pressureof oxygen in thelungsor in muscle. In hemoglobin the four subunits show a cooperativity effect that allows for easy oxygen transfer from hemoglobin to myoglobin.[11]
In bothhemoglobinandmyoglobinit is sometimes incorrectly stated that the oxygenated species contains iron(III). It is now known that thediamagneticnature of these species is because the iron(II) atom is in thelow-spinstate. Inoxyhemoglobinthe iron atom is located in the plane of the porphyrin ring, but in theparamagneticdeoxyhemoglobinthe iron atom lies above the plane of the ring.[11]This change in spin state is a cooperative effect due to the highercrystal field splittingand smallerionic radiusof Fe2+in the oxyhemoglobin moiety.
Hemerythrinis another iron-containing oxygen carrier. The oxygen binding site is a binuclear iron center. The iron atoms are coordinated to the protein through thecarboxylateside chains of aglutamateandaspartateand fivehistidineresidues. The uptake of O2by hemerythrin is accompanied by two-electron oxidation of the reduced binuclear center to produce boundperoxide(OOH−). The mechanism of oxygen uptake and release have been worked out in detail.[12][13]
Hemocyaninscarry oxygen in the blood of mostmollusks,and somearthropodssuch as thehorseshoe crab.They are second only to hemoglobin in biological popularity of use in oxygen transport. On oxygenation the twocopper(I) atoms at the active site are oxidized to copper(II) and the dioxygen molecules are reduced to peroxide,O2−
2.[14][15]
Chlorocruorin(as the larger carriererythrocruorin) is an oxygen-binding hemeprotein present in theblood plasmaof manyannelids,particularly certain marinepolychaetes.
Cytochromes
[edit]Oxidationandreductionreactions are not common inorganic chemistryas few organic molecules can act asoxidizingorreducing agents.Iron(II), on the other hand, can easily be oxidized to iron(III). This functionality is used incytochromes,which function aselectron-transfervectors. The presence of the metal ion allowsmetalloenzymesto perform functions such asredox reactionsthat cannot easily be performed by the limited set offunctional groupsfound inamino acids.[16]The iron atom in most cytochromes is contained in ahemegroup. The differences between those cytochromes lies in the different side-chains. For instance cytochrome a has aheme aprosthetic group and cytochrome b has aheme bprosthetic group. These differences result in different Fe2+/Fe3+redox potentialssuch that various cytochromes are involved in themitochondrialelectron transport chain.[17]
Cytochrome P450enzymes perform the function of inserting an oxygen atom into a C−H bond, an oxidation reaction.[18][19]
Rubredoxin
[edit]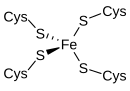
Rubredoxinis an electron-carrier found insulfur-metabolizingbacteriaandarchaea.The active site contains an iron ion coordinated by the sulfur atoms of fourcysteineresidues forming an almost regulartetrahedron.Rubredoxins perform one-electron transfer processes. Theoxidation stateof the iron atom changes between the +2 and +3 states. In both oxidation states the metal ishigh spin,which helps to minimize structural changes.
Plastocyanin
[edit]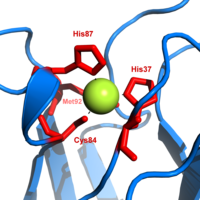
Plastocyanin is one of the family of bluecopper proteinsthat are involved inelectron transferreactions. Thecopper-binding site is described as distortedtrigonal pyramidal.[20]The trigonal plane of the pyramidal base is composed of two nitrogen atoms (N1and N2) from separate histidines and a sulfur (S1) from a cysteine. Sulfur (S2) from an axial methionine forms the apex. The distortion occurs in the bond lengths between the copper and sulfur ligands. The Cu−S1contact is shorter (207pm) than Cu−S2(282 pm). The elongated Cu−S2bonding destabilizes the Cu(II) form and increases theredox potentialof the protein. The blue color (597nmpeak absorption) is due to the Cu−S1bond where S(pπ) to Cu(dx2−y2) charge transfer occurs.[21]
In the reduced form of plastocyanin,His-87 will become protonated with apKaof 4.4.Protonationprevents it acting as aligandand the copper site geometry becomestrigonal planar.
Metal-ion storage and transfer
[edit]Iron
[edit]Ironis stored as iron(III) inferritin.The exact nature of the binding site has not yet been determined. The iron appears to be present as ahydrolysisproduct such as FeO(OH). Iron is transported bytransferrinwhose binding site consists of twotyrosines,oneaspartic acidand onehistidine.[22]The human body has no controlled mechanism for excretion of iron.[23]This can lead toiron overloadproblems in patients treated withblood transfusions,as, for instance, with β-thalassemia.Iron is actually excreted in urine[24]and is also concentrated in bile[25]which is excreted in feces.[26]
Copper
[edit]Ceruloplasmin is the majorcopper-carrying protein in the blood. Ceruloplasmin exhibits oxidase activity, which is associated with possible oxidation of Fe(II) into Fe(III), therefore assisting in its transport in theblood plasmain association with transferrin, which can carry iron only in the Fe(III) state.
Calcium
[edit]Osteopontin is involved in mineralization in the extracellular matrices of bones and teeth.
Metalloenzymes
[edit]Metalloenzymes all have one feature in common, namely that the metal ion is bound to the protein with onelabilecoordinationsite. As with allenzymes,the shape of theactive siteis crucial. The metal ion is usually located in a pocket whose shape fits the substrate. The metal ioncatalyzesreactions that are difficult to achieve inorganic chemistry.
Carbonic anhydrase
[edit]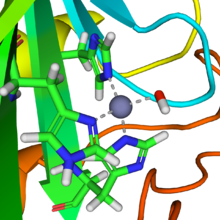
Inaqueous solution,carbon dioxideformscarbonic acid
- CO2+ H2O ⇌ H2CO3
This reaction is very slow in the absence of a catalyst, but quite fast in the presence of thehydroxideion
- CO2+ OH−⇌HCO−
3
A reaction similar to this is almost instantaneous withcarbonic anhydrase.The structure of the active site in carbonic anhydrases is well known from a number of crystal structures. It consists of azincion coordinated by threeimidazolenitrogen atoms from threehistidineunits. The fourth coordination site is occupied by a water molecule. The coordination sphere of the zinc ion is approximatelytetrahedral.The positively-charged zinc ion polarizes the coordinated water molecule, andnucleophilicattack by the negatively-charged hydroxide portion on carbon dioxide proceeds rapidly. The catalytic cycle produces the bicarbonate ion and the hydrogen ion[2]as theequilibrium:
- H2CO3⇌HCO−
3+ H+
favouring dissociation ofcarbonic acidat biologicalpHvalues.[27]
Vitamin B12-dependent enzymes
[edit]Thecobalt-containingVitamin B12(also known as cobalamin) catalyzes the transfer ofmethyl(−CH3) groups between two molecules, which involves the breaking ofC−C bonds,a process that is energetically expensive in organic reactions. The metal ion lowers theactivation energyfor the process by forming a transient Co−CH3bond.[28]The structure of thecoenzymewas famously determined byDorothy Hodgkinand co-workers, for which she received aNobel Prize in Chemistry.[29]It consists of a cobalt(II) ion coordinated to four nitrogen atoms of acorrinring and a fifth nitrogen atom from animidazolegroup. In the resting state there is a Co−Csigma bondwith the 5′ carbon atom ofadenosine.[30]This is a naturally occurringorganometalliccompound, which explains its function intrans-methylation reactions, such as the reaction carried out bymethionine synthase.
Nitrogenase (nitrogen fixation)
[edit]Thefixation of atmospheric nitrogenis an energy-intensive process, as it involves breaking the very stabletriple bondbetween the nitrogen atoms. Thenitrogenasescatalyze the process. One such enzyme occurs inRhizobiumbacteria.There are three components to its action: amolybdenumatom at the active site,iron–sulfur clustersthat are involved in transporting the electrons needed to reduce the nitrogen, and an abundant energy source in the form ofmagnesiumATP.This last is provided by amutualistic symbiosisbetween the bacteria and a host plant, often alegume.The reaction may be written symbolically as
where Pistands for inorganicphosphate.The precise structure of the active site has been difficult to determine. It appears to contain a MoFe7S8cluster that is able to bind the dinitrogen molecule and, presumably, enable the reduction process to begin.[31]The electrons are transported by the associated "P" cluster, which contains twocubicalFe4S4clusters joined by sulfur bridges.[32]
Superoxide dismutase
[edit]
Thesuperoxideion,O−
2is generated in biological systems by reduction of molecularoxygen.It has an unpairedelectron,so it behaves as afree radical.It is a powerfuloxidizing agent.These properties render the superoxide ion verytoxicand are deployed to advantage byphagocytesto kill invadingmicroorganisms.Otherwise, the superoxide ion must be destroyed before it does unwanted damage in a cell. Thesuperoxide dismutaseenzymes perform this function very efficiently.[33]
The formaloxidation stateof the oxygen atoms is −1⁄2.In solutions at neutralpH,the superoxide iondisproportionatesto molecular oxygen andhydrogen peroxide.
- 2O−
2+ 2 H+→ O2+ H2O2
In biology this type of reaction is called adismutationreaction. It involves both oxidation and reduction of superoxide ions. Thesuperoxide dismutase(SOD) group of enzymes increase therate of reactionto near the diffusion-limited rate.[34]The key to the action of these enzymes is a metal ion with variable oxidation state that can act either as an oxidizing agent or as a reducing agent.
- Oxidation: M(n+1)++O−
2→ Mn++ O2 - Reduction: Mn++O−
2+ 2 H+→ M(n+1)++ H2O2.
In human SOD, the active metal iscopper,as Cu(II) or Cu(I), coordinatedtetrahedrallyby fourhistidineresidues. This enzyme also containszincions for stabilization and is activated by copper chaperone for superoxide dismutase (CCS). Otherisozymesmay containiron,manganese ornickel.The activity of Ni-SOD involves nickel(III), an unusual oxidation state for this element. The active site nickel geometry cycles fromsquare planarNi(II), with thiolate (Cys2and Cys6) and backbone nitrogen (His1and Cys2) ligands, tosquare pyramidalNi(III) with an added axial His1side chain ligand.[35]
Chlorophyll-containing proteins
[edit]
Chlorophyll plays a crucial role inphotosynthesis.It contains amagnesiumenclosed in achlorinring. However, the magnesium ion is not directly involved in the photosynthetic function and can be replaced by other divalent ions with little loss of activity. Rather, thephotonis absorbed by the chlorin ring, whose electronic structure is well-adapted for this purpose.
Initially, the absorption of a photon causes anelectronto be excited into asinglet stateof the Q band. Theexcited stateundergoes anintersystem crossingfrom the singlet state to atriplet statein which there are two electrons with parallelspin.This species is, in effect, afree radical,and is very reactive and allows an electron to be transferred to acceptors that are adjacent to the chlorophyll in thechloroplast.In the process chlorophyll is oxidized. Later in the photosynthetic cycle, chlorophyll is reduced back again. This reduction ultimately draws electrons from water, yielding molecular oxygen as a final oxidation product.
Hydrogenase
[edit]Hydrogenases are subclassified into three different types based on the active site metal content: iron–iron hydrogenase, nickel–iron hydrogenase, and iron hydrogenase.[36] All hydrogenases catalyze reversibleH2uptake, but while the [FeFe] and [NiFe] hydrogenases are trueredoxcatalysts,driving H2oxidation and H+reduction
- H2⇌ 2 H++ 2 e−
the [Fe] hydrogenases catalyze the reversibleheterolyticcleavage of H2.
- H2⇌ H++ H−
Ribozyme and deoxyribozyme
[edit]Since discovery ofribozymesbyThomas CechandSidney Altmanin the early 1980s, ribozymes have been shown to be a distinct class of metalloenzymes.[37]Many ribozymes require metal ions in their active sites for chemical catalysis; hence they are called metalloenzymes. Additionally, metal ions are essential for structural stabilization of ribozymes.Group I intronis the most studied ribozyme which has three metals participating in catalysis.[38]Other known ribozymes includegroup II intron,RNase P,and several small viral ribozymes (such ashammerhead,hairpin,HDV,andVS) and the large subunit of ribosomes. Several classes of ribozymes have been described.[39]
Deoxyribozymes,also called DNAzymes or catalytic DNA, are artificial DNA-based catalysts that were first produced in 1994.[40]Almost all DNAzymes require metal ions. Although ribozymes mostly catalyze cleavage of RNA substrates, a variety of reactions can be catalyzed by DNAzymes including RNA/DNA cleavage, RNA/DNA ligation, amino acid phosphorylation and dephosphorylation, and carbon–carbon bond formation.[41]Yet, DNAzymes that catalyze RNA cleavage reaction are the most extensively explored ones. 10-23 DNAzyme, discovered in 1997, is one of the most studied catalytic DNAs with clinical applications as a therapeutic agent.[42]Several metal-specific DNAzymes have been reported including the GR-5 DNAzyme (lead-specific),[43]the CA1-3 DNAzymes (copper-specific), the 39E DNAzyme (uranyl-specific)[44]and the NaA43 DNAzyme (sodium-specific).[45]
Signal-transduction metalloproteins
[edit]Calmodulin
[edit]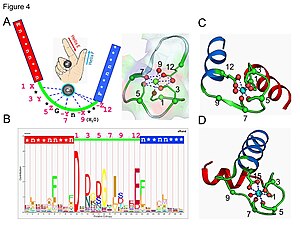
Calmodulinis an example of a signal-transduction protein. It is a small protein that contains fourEF-handmotifs, each of which is able to bind aCa2+ion.
In anEF-handloop protein domain, the calcium ion is coordinated in a pentagonal bipyramidal configuration. Sixglutamic acidandaspartic acidresidues involved in the binding are in positions 1, 3, 5, 7 and 9 of the polypeptide chain. At position 12, there is a glutamate or aspartate ligand that behaves as abidentateligand, providing two oxygen atoms. The ninth residue in the loop is necessarilyglycinedue to the conformational requirements of the backbone. Thecoordination sphereof the calcium ion contains only carboxylate oxygen atoms and no nitrogen atoms. This is consistent with thehardnature of the calcium ion.
The protein has two approximately symmetrical domains, separated by a flexible "hinge" region. Binding of calcium causes a conformational change to occur in the protein. Calmodulin participates in anintracellular signalingsystem by acting as a diffusible second messenger to the initial stimuli.[46][47]
Troponin
[edit]In bothcardiacandskeletal muscles,muscular force production is controlled primarily by changes in the intracellularcalciumconcentration.In general, when calcium rises, the muscles contract and, when calcium falls, the muscles relax.Troponin,along withactinandtropomyosin,is the protein complex to which calcium binds to trigger the production of muscular force.
Transcription factors
[edit]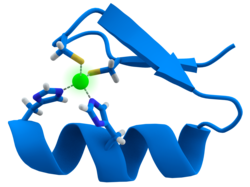
Manytranscription factorscontain a structure known as azinc finger,a structural module in which a region of protein folds around a zinc ion. The zinc does not directly contact theDNAthat these proteins bind to. Instead, the cofactor is essential for the stability of the tightly folded protein chain.[48]In these proteins, the zinc ion is usually coordinated by pairs of cysteine and histidine side-chains.
Other metalloenzymes
[edit]There are two types ofcarbon monoxide dehydrogenase:one contains iron and molybdenum, the other contains iron and nickel. Parallels and differences in catalytic strategies have been reviewed.[49]
Pb2+(lead) can replace Ca2+(calcium) as, for example, withcalmodulinor Zn2+(zinc) as withmetallocarboxypeptidases.[50]
Some other metalloenzymes are given in the following table, according to the metal involved.
See also
[edit]References
[edit]- ^Banci L (2013). "Metallomics and the Cell: Some Definitions and General Comments". In Sigel A, Sigel H, Sigel RK (eds.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer. pp. 1–13.doi:10.1007/978-94-007-5561-1_1.ISBN978-94-007-5561-1.PMID23595668.
- ^abShriver DF, Atkins PW (1999). "Charper 19, Bioinorganic chemistry".Inorganic chemistry(3rd ed.). Oxford University Press.ISBN978-0-19-850330-9.
- ^Human reference proteomein Uniprot, accessed 12 Jan 2018
- ^Andreini C, Banci L, Bertini I, Rosato A (November 2006). "Zinc through the three domains of life".Journal of Proteome Research.5(11): 3173–8.doi:10.1021/pr0603699.PMID17081069.
- ^Thomson AJ, Gray HB (1998)."Bioinorganic chemistry"(PDF).Current Opinion in Chemical Biology.2(2): 155–158.doi:10.1016/S1367-5931(98)80056-2.PMID9667942.
- ^Waldron KJ, Robinson NJ (January 2009). "How do bacterial cells ensure that metalloproteins get the correct metal?".Nature Reviews. Microbiology.7(1): 25–35.doi:10.1038/nrmicro2057.PMID19079350.S2CID7253420.
- ^Carver PL (2013). "Metal Ions and Infectious Diseases. An Overview from the Clinic". In Sigel A, Sigel H, Sigel RK (eds.).Interrelations between Essential Metal Ions and Human Diseases.Metal Ions in Life Sciences. Vol. 13. Springer. pp. 1–28.doi:10.1007/978-94-007-7500-8_1.ISBN978-94-007-7499-5.PMID24470087.
- ^Wang, MS; Hoegler, KH; Hecht, M (2019)."Unevolved De Novo Proteins Have Innate Tendencies to Bind Transition Metals".Life.9(8): 8.Bibcode:2019Life....9....8W.doi:10.3390/life9010008.PMC6463171.PMID30634485.
- ^Maret W (February 2010)."Metalloproteomics, metalloproteomes, and the annotation of metalloproteins".Metallomics.2(2): 117–25.doi:10.1039/b915804a.PMID21069142.
- ^Cangelosi V, Ruckthong L, Pecoraro VL (2017). "Chapter 10. Lead(II) Binding in Natural and Artificial Proteins". In Astrid S, Helmut S, Sigel RK (eds.).Lead: Its Effects on Environment and Health.Metal Ions in Life Sciences. Vol. 17. de Gruyter. pp. 271–318.doi:10.1515/9783110434330-010.ISBN9783110434330.PMC5771651.PMID28731303.
- ^abGreenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.Fig.25.7, p 1100 illustrates the structure of deoxyhemoglobin
- ^Stenkamp, R. E. (1994). "Dioxygen and hemerythrin".Chem. Rev.94(3): 715–726.doi:10.1021/cr00027a008.
- ^Wirstam M, Lippard SJ, Friesner RA (April 2003). "Reversible dioxygen binding to hemerythrin".Journal of the American Chemical Society.125(13): 3980–7.doi:10.1021/ja017692r.PMID12656634.
- ^Karlin K, Cruse RW, Gultneh Y, Farooq A, Hayes JC, Zubieta J (1987). "Dioxygen–copper reactivity. Reversible binding of O2and CO to a phenoxo-bridged dicopper(I) complex ".J. Am. Chem. Soc.109(9): 2668–2679.doi:10.1021/ja00243a019.
- ^Kitajima N, Fujisawa K, Fujimoto C, Morooka Y, Hashimoto S, Kitagawa T, Toriumi K, Tatsumi K, Nakamura A (1992). "A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of theμ-η2:η2-peroxo dinuclear copper(II) complexes, [Cu(Hb(3,5-R2pz)3)]2(O2) (R = isopropyl and Ph) ".J. Am. Chem. Soc.114(4): 1277–1291.doi:10.1021/ja00030a025.
- ^Messerschmidt A, Huber R, Wieghardt K, Poulos T (2001).Handbook of Metalloproteins.Wiley.ISBN978-0-471-62743-2.
- ^Moore GR, Pettigrew GW (1990).Cytochrome c: Structural and Physicochemical Aspects.Berlin: Springer.
- ^Sigel A, Sigel H, Sigel RK, eds. (2007).The Ubiquitous Roles of Cytochrome 450 Proteins.Metal Ions in Life Sciences. Vol. 3. Wiley.ISBN978-0-470-01672-5.
- ^Ortiz de Montellano P (2005).Cytochrome P450 Structure, Mechanism, and Biochemistry(3rd ed.). Springer.ISBN978-0-306-48324-0.
- ^Colman PM,Freeman HC,Guss JM, Murata M, Norris VA, Ramshaw JA, Venkatappa MP (1978). "X-Ray Crystal-Structure Analysis of Plastocyanin at 2.7 Å Resolution".Nature.272(5651): 319–324.Bibcode:1978Natur.272..319C.doi:10.1038/272319a0.S2CID4226644.
- ^Solomon EI, Gewirth AA, Cohen SL (1986).Spectroscopic Studies of Active Sites. Blue Copper and Electronic Structural Analogs.Vol. 307. pp. 236–266.doi:10.1021/bk-1986-0307.ch016.ISBN978-0-8412-0971-8.
{{cite book}}:|journal=ignored (help) - ^Anderson BF, Baker HM, Dodson EJ, Norris GE, Rumball SV, Waters JM, Baker EN (April 1987)."Structure of human lactoferrin at 3.2-A resolution".Proceedings of the National Academy of Sciences of the United States of America.84(7): 1769–73.doi:10.1073/pnas.84.7.1769.PMC304522.PMID3470756.
- ^Wallace, Daniel F (May 2016)."The Regulation of Iron Absorption and Homeostasis".The Clinical Biochemist Reviews.37(2): 51–62.ISSN0159-8090.PMC5198508.PMID28303071.
- ^Rodríguez E, Díaz C (December 1995). "Iron, copper and zinc levels in urine: relationship to various individual factors".Journal of Trace Elements in Medicine and Biology.9(4): 200–9.Bibcode:1995JTEMB...9..200R.doi:10.1016/S0946-672X(11)80025-8.PMID8808191.
- ^Schümann K, Schäfer SG, Forth W (1986). "Iron absorption and biliary excretion of transferrin in rats".Research in Experimental Medicine. Zeitschrift für die Gesamte Experimentelle Medizin Einschliesslich Experimenteller Chirurgie.186(3): 215–9.doi:10.1007/BF01852047.PMID3738220.S2CID7925719.
- ^"Biliary excretion of waste products".Archived fromthe originalon 2017-03-26.Retrieved2017-03-24.
- ^Lindskog S (1997). "Structure and mechanism of carbonic anhydrase".Pharmacology & Therapeutics.74(1): 1–20.doi:10.1016/S0163-7258(96)00198-2.PMID9336012.
- ^Sigel A, Sigel H, Sigel RK, eds. (2008).Metal–carbon bonds in enzymes and cofactors.Metal Ions in Life Sciences. Vol. 6. Wiley.ISBN978-1-84755-915-9.
- ^"The Nobel Prize in Chemistry 1964".Nobelprize.org.Retrieved2008-10-06.
- ^Hodgkin, D. C. (1965). "The Structure of the Corrin Nucleus from X-ray Analysis".Proc. R. Soc. A.288(1414): 294–305.Bibcode:1965RSPSA.288..294H.doi:10.1098/rspa.1965.0219.S2CID95235740.
- ^Orme-Johnson, W. H. (1993). Steifel, E. I.; Coucouvannis, D.; Newton, D. C. (eds.).Molybdenum enzymes, cofactors and model systems.Advances in chemistry, Symposium series no. 535. Washington, DC: American Chemical Society. pp.257.ISBN9780841227088.
- ^Chan MK, Kim J, Rees DC (May 1993). "The nitrogenase FeMo-cofactor and P-cluster pair: 2.2 A resolution structures".Science.260(5109): 792–4.doi:10.1126/science.8484118.PMID8484118.
- ^Packer, L., ed. (2002).Superoxide Dismutase: 349 (Methods in Enzymology).Academic Press.ISBN978-0-12-182252-1.
- ^Heinrich P, Löffler G, Petrides PE (2006).Biochemie und Pathobiochemie(in German). Berlin: Springer. p. 123.ISBN978-3-540-32680-9.
- ^Barondeau DP, Kassmann CJ, Bruns CK, Tainer JA,Getzoff ED(June 2004). "Nickel superoxide dismutase structure and mechanism".Biochemistry.43(25): 8038–47.doi:10.1021/bi0496081.PMID15209499.
- ^ Parkin, Alison (2014). "Understanding and Harnessing Hydrogenases, Biological Dihydrogen Catalysts". In Kroneck, Peter M. H.; Sosa Torres, Martha E. (eds.).The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment.Metal Ions in Life Sciences. Vol. 14. Springer. pp. 99–124.doi:10.1007/978-94-017-9269-1_5.ISBN978-94-017-9268-4.PMID25416392.
- ^Pyle AM (August 1993). "Ribozymes: a distinct class of metalloenzymes".Science.261(5122): 709–14.Bibcode:1993Sci...261..709P.doi:10.1126/science.7688142.PMID7688142.
- ^Shan S, Yoshida A, Sun S, Piccirilli JA, Herschlag D (October 1999)."Three metal ions at the active site of the Tetrahymena group I ribozyme".Proceedings of the National Academy of Sciences of the United States of America.96(22): 12299–304.Bibcode:1999PNAS...9612299S.doi:10.1073/pnas.96.22.12299.PMC22911.PMID10535916.
- ^Weinberg Z, Kim PB, Chen TH, Li S, Harris KA, Lünse CE, Breaker RR (August 2015)."New classes of self-cleaving ribozymes revealed by comparative genomics analysis".Nature Chemical Biology.11(8): 606–10.doi:10.1038/nchembio.1846.PMC4509812.PMID26167874.
- ^Breaker RR, Joyce GF (December 1994). "A DNA enzyme that cleaves RNA".Chemistry & Biology.1(4): 223–9.doi:10.1016/1074-5521(94)90014-0.PMID9383394.
- ^Silverman SK (May 2015)."Pursuing DNA catalysts for protein modification".Accounts of Chemical Research.48(5): 1369–79.doi:10.1021/acs.accounts.5b00090.PMC4439366.PMID25939889.
- ^Santoro SW, Joyce GF (April 1997)."A general purpose RNA-cleaving DNA enzyme".Proceedings of the National Academy of Sciences of the United States of America.94(9): 4262–6.Bibcode:1997PNAS...94.4262S.doi:10.1073/pnas.94.9.4262.PMC20710.PMID9113977.
- ^Breaker RR, Joyce GF (December 1994). "A DNA enzyme that cleaves RNA".Chemistry & Biology.1(4): 223–9.doi:10.1016/1074-5521(94)90014-0.PMID9383394.
- ^Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y (February 2007)."A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity".Proceedings of the National Academy of Sciences of the United States of America.104(7): 2056–61.Bibcode:2007PNAS..104.2056L.doi:10.1073/pnas.0607875104.PMC1892917.PMID17284609.
- ^Torabi SF, Wu P, McGhee CE, Chen L, Hwang K, Zheng N, Cheng J, Lu Y (May 2015)."In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing".Proceedings of the National Academy of Sciences of the United States of America.112(19): 5903–8.Bibcode:2015PNAS..112.5903T.doi:10.1073/pnas.1420361112.PMC4434688.PMID25918425.
- ^Stevens FC (August 1983). "Calmodulin: an introduction".Canadian Journal of Biochemistry and Cell Biology.61(8): 906–10.doi:10.1139/o83-115.PMID6313166.
- ^Chin D, Means AR (August 2000). "Calmodulin: a prototypical calcium sensor".Trends in Cell Biology.10(8): 322–8.doi:10.1016/S0962-8924(00)01800-6.PMID10884684.
- ^Berg JM (1990). "Zinc finger domains: hypotheses and current knowledge".Annual Review of Biophysics and Biophysical Chemistry.19(1): 405–21.doi:10.1146/annurev.bb.19.060190.002201.PMID2114117.
- ^Jeoung JH, Fesseler J, Goetzl S, Dobbek H (2014). "Carbon Monoxide. Toxic Gas and Fuel for Anaerobes and Aerobes: Carbon Monoxide Dehydrogenases". In Kroneck PM, Sosa Torres ME (eds.).The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment.Metal Ions in Life Sciences. Vol. 14. Springer. pp. 37–69.doi:10.1007/978-94-017-9269-1_3.ISBN978-94-017-9268-4.PMID25416390.
- ^Aoki K, Murayama K, Hu NH (2017). "Chapter 7. Solid State Structures of Lead Complexes with Relevance for Biological Systems". In Astrid S, Helmut S, Sigel RK (eds.).Lead: Its Effects on Environment and Health.Metal Ions in Life Sciences. Vol. 17. de Gruyter. pp. 123–200.doi:10.1515/9783110434330-007.ISBN9783110434330.PMID28731300.
- ^Romani, Andrea M. P. (2013). "Magnesium Homeostasis in Mammalian Cells". In Banci, Lucia (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer. pp. 69–118.doi:10.1007/978-94-007-5561-1_4.ISBN978-94-007-5561-1.ISSN1868-0402.PMID23595671.
- ^Roth J, Ponzoni S, Aschner M (2013). "Manganese Homeostasis and Transport". In Banci L (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer. pp. 169–201.doi:10.1007/978-94-007-5561-1_6.ISBN978-94-007-5561-1.ISSN1868-0402.PMC6542352.PMID23595673.
- ^Dlouhy AC, Outten CE (2013). "The Iron Metallome in Eukaryotic Organisms". In Banci L (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer. pp. 241–78.doi:10.1007/978-94-007-5561-1_8.ISBN978-94-007-5561-1.ISSN1868-0402.PMC3924584.PMID23595675.
- ^Cracan V, Banerjee R (2013). "Chapter 10 Cobalt and Corrinoid Transport and Biochemistry". In Banci L (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer.doi:10.1007/978-94-007-5561-10_10(inactive 2024-09-12).ISBN978-94-007-5561-1.ISSN1868-0402.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^Sigel A, Sigel H, Sigel RK, eds. (2008).Nickel and Its Surprising Impact in Nature.Metal Ions in Life Sciences. Vol. 2. Wiley.ISBN978-0-470-01671-8.
- ^Sydor AM, Zambie DB (2013). "Chapter 11. Nickel Metallomics: General Themes Guiding Nickel Homeostasis". In Banci L (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer.doi:10.1007/978-94-007-5561-10_11(inactive 2024-09-12).ISBN978-94-007-5561-1.ISSN1868-0402.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^Vest KE, Hashemi HF, Cobine PA (2013). "Chapter 13. The Copper Metallome in Eukaryotic Cells". In Banci L (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer.doi:10.1007/978-94-007-5561-10_12(inactive 2024-09-12).ISBN978-94-007-5561-1.ISSN1868-0402.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^Maret W (2013). "Chapter 14 Zinc and the Zinc Proteome". In Banci L (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer.doi:10.1007/978-94-007-5561-10_14(inactive 2024-09-12).ISBN978-94-007-5561-1.ISSN1868-0402.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^Peacock AF, Pecoraro V (2013). "Natural and Artificial Proteins Containing Cadmium". In Sigel A, Sigel H, Sigel RK (eds.).Cadmium: From Toxicity to Essentiality.Metal Ions in Life Sciences. Vol. 11. Springer. pp. 303–337.doi:10.1007/978-94-007-5179-8_10.ISBN978-94-007-5178-1.PMID23430777.
- ^Freisinger EF, Vasac M (2013). "Cadmium in Metallothioneins". In Sigel A, Sigel H, Sigel RK (eds.).Cadmium: From Toxicity to Essentiality.Metal Ions in Life Sciences. Vol. 11. Springer. pp. 339–372.doi:10.1007/978-94-007-5179-8_11.ISBN978-94-007-5178-1.PMID23430778.
- ^Mendel, Ralf R. (2013). "Chapter 15. Metabolism of Molybdenum". In Banci, Lucia (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer.doi:10.1007/978-94-007-5561-10_15(inactive 2024-09-12).ISBN978-94-007-5561-1.ISSN1868-0402.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^ten Brink, Felix (2014). "Living on Acetylene. A Primordial Energy Source". In Kroneck, Peter M. H.; Sosa Torres, Martha E. (eds.).The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment.Metal Ions in Life Sciences. Vol. 14. Springer. pp. 15–35.doi:10.1007/978-94-017-9269-1_2.ISBN978-94-017-9268-4.PMID25416389.
External links
[edit]- Metalloproteinat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- Catherine Drennan's Seminar: Snapshots of Metalloproteins
