From Wikipedia, the free encyclopedia
Chemical compound
Methallenestril Trade names Cur-men, Ercostrol, Geklimon, Novestrine, Vallestril (also spelled Vallestrol or Vallestryl) Other names Methallenoestril; Methallenestrol; Methallenoestrol; Horeau's acid; Allenestrol 6-methyl ether; α,α-Dimethyl-β-ethylallenolic acid 6-methyl ether; β-Ethyl-6-methoxy-α,α-dimethyl-2-naphthalenepropionic acid Routes of By mouth Drug class Nonsteroidal estrogen ATC code
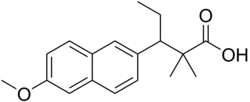
3-(6-Methoxynaphthalen-2-yl)-2,2-dimethylpentanoic acid
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) ECHA InfoCard 100.007.485 Formula C 18 H 22 O 3 Molar mass −1 3D model (JSmol )
CCC(c1ccc2cc(OC)ccc2c1)C(C)(C)C(=O)O
InChI=1S/C18H22O3/c1-5-16(18(2,3)17(19)20)14-7-6-13-11-15(21-4)9-8-12(13)10-14/h6-11,16H,5H2,1-4H3,(H,19,20)
Key:KHLJKRBMZVNZOC-UHFFFAOYSA-N
(verify)
Methallenestril (INN Tooltip International Nonproprietary Name ) (brand namesCur-men ,Ercostrol ,Geklimon ,Novestrine ,Vallestril ), also known asmethallenoestril (BAN Tooltip British Approved Name ) and asmethallenestrol ,as well asHoreau's acid ,[ 1] [ 2] synthetic nonsteroidal estrogen and aderivative ofallenolic acid andallenestrol (specifically, amethyl ether of it) that was formerly used to treatmenstrual issues but is now no longer marketed.[ 3] [ 4] [ 5] [ 6] analogue ofbisdehydrodoisynolic acid ,and although methallenestril is potentlyestrogenic in rats, in humans it is only weakly so in comparison.[ 7] Vallestril was a brand of methallenestril issued byG. D. Searle & Company in the 1950s.[ 8] by mouth .[ 9] diethylstilbestrol ,4 mgdienestrol ,20 mghexestrol ,25 mgestrone ,2.5 mgconjugated estrogens ,and 0.05 mgethinylestradiol .[ 9]
Estrogens
ER Tooltip Estrogen receptor agonists
Steroidal: Alfatradiol Certainandrogens /anabolic steroids (e.g.,testosterone ,testosterone esters ,methyltestosterone ,metandienone ,nandrolone esters ) (via estrogenic metabolites)
Certainprogestins (e.g.,norethisterone ,noretynodrel ,etynodiol diacetate ,tibolone )
Clomestrone Cloxestradiol acetate Conjugated estriol Conjugated estrogens Epiestriol Epimestrol Esterified estrogens Estetrol † Estradiol Estradiol esters (e.g.,estradiol acetate ,estradiol benzoate ,estradiol cypionate ,estradiol enanthate ,estradiol undecylate ,estradiol valerate ,polyestradiol phosphate ,estradiol ester mixtures (Climacteron ))Estramustine phosphate Estriol Estriol esters (e.g.,estriol succinate ,polyestriol phosphate )Estrogenic substances Estrone Estrone esters
Ethinylestradiol #
Hydroxyestrone diacetate Mestranol Methylestradiol Moxestrol Nilestriol Prasterone (dehydroepiandrosterone; DHEA)
Promestriene Quinestradol Quinestrol Progonadotropins
Antiestrogens
ER Tooltip Estrogen receptor antagonistsSERMs Tooltip selective estrogen receptor modulators /SERDs Tooltip selective estrogen receptor downregulators )Aromatase inhibitors Antigonadotropins
Androgens /anabolic steroids (e.g.,testosterone ,testosterone esters ,nandrolone esters ,oxandrolone ,fluoxymesterone )D2 receptor antagonists (prolactin releasers) (e.g.,domperidone ,metoclopramide ,risperidone ,haloperidol ,chlorpromazine ,sulpiride )GnRH agonistsleuprorelin ,goserelin )GnRH antagonistscetrorelix ,elagolix )Progestogens (e.g.,chlormadinone acetate ,cyproterone acetate ,gestonorone caproate ,hydroxyprogesterone caproate ,medroxyprogesterone acetate ,megestrol acetate ) Others
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g.,testosterone andesters ,methyltestosterone ,metandienone (methandrostenolone) ,nandrolone andesters ,many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g.,anethole ,anol ,dianethole ,dianol ,photoanethole )Chalconoids (e.g.,isoliquiritigenin ,phloretin ,phlorizin (phloridzin) ,wedelolactone )Coumestans (e.g.,coumestrol ,psoralidin )Flavonoids (incl.7,8-DHF ,8-prenylnaringenin ,apigenin ,baicalein ,baicalin ,biochanin A ,calycosin ,catechin ,daidzein ,daidzin ,ECG ,EGCG ,epicatechin ,equol ,formononetin ,glabrene ,glabridin ,genistein ,genistin ,glycitein ,kaempferol ,liquiritigenin ,mirificin ,myricetin ,naringenin ,penduletin ,pinocembrin ,prunetin ,puerarin ,quercetin ,tectoridin ,tectorigenin )Lavender oil Lignans (e.g.,enterodiol ,enterolactone ,nyasol (cis -hinokiresinol) )Metalloestrogens (e.g.,cadmium )Pesticides (e.g.,alternariol ,dieldrin ,endosulfan ,fenarimol ,HPTE ,methiocarb ,methoxychlor ,triclocarban ,triclosan )Phytosteroids (e.g.,digitoxin (digitalis ),diosgenin ,guggulsterone )Phytosterols (e.g.,β-sitosterol ,campesterol ,stigmasterol )Resorcylic acid lactones (e.g.,zearalanone ,α-zearalenol ,β-zearalenol ,zearalenone ,zeranol (α-zearalanol) ,taleranol (teranol, β-zearalanol) )Steroid -like (e.g.,deoxymiroestrol ,miroestrol )Stilbenoids (e.g.,resveratrol ,rhaponticin )Synthetic xenoestrogens (e.g.,alkylphenols ,bisphenols (e.g.,BPA ,BPF ,BPS ),DDT ,parabens ,PBBs ,PHBA ,phthalates ,PCBs )Others (e.g.,agnuside ,rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown