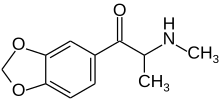
Methylone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3,4-Methylenedioxy-N-methylcathinone; Methylenedioxymethcathinone; MDMC; β-Keto-MDMA; βk-MDMA; M1 |
| Routes of administration | Common:oral,insufflation Uncommon:IVorIMinjection,rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokineticdata | |
| Onset of action | 0.5 hours[3] |
| Eliminationhalf-life | 5.8–6.9 hours[3] |
| Duration of action | 2.5–3.0 hours[3] |
| Identifiers | |
| |
| CAS Number |
|
| PubChemCID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C11H13NO3 |
| Molar mass | 207.229g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 357 mg/mL (20 °C) |
| |
| |
Methylone,also known as3,4-methylenedioxy-N-methylcathinone(MDMC), is anempathogenandstimulantpsychoactive drug.It is a member of theamphetamine,cathinoneandmethylenedioxyphenethylamineclasses.
Methylone is a slight modification of3,4-methylenedioxymethamphetamine(MDMA, also known as ecstasy). It was first synthesized by the chemists Peyton Jacob III andAlexander Shulginin 1996 for potential use as an antidepressant.[4]Methylone has been sold forrecreational use,taking advantage of the absence of legal prohibition of this compound in many countries.[citation needed]
Chemistry[edit]
Methylone is the substituted cathinoneanalogueof 3,4-methylenedioxymethamphetamine (MDMA) and the 3,4-methylenedioxyanalog ofmethcathinone.The only structural difference of methylone with respect to MDMA is the substitution of 2 hydrogen atoms by 1 oxygen atom in the β position of thephenethylaminecore, forming aketonegroup.[5]
Effects[edit]
Resemblance to MDMA[edit]

Left:amphetamine,methamphetamineandmethcathinone.
Right:MDA,MDMA,and methylone
Methylone substitutes for MDMA in rats trained to discriminate MDMA fromsaline.Methylone does not substitute for amphetamine or for the hallucinogenic DOM in animals trained to discriminate between these drugs and saline.[6]Further, also in common with MDMA, methylone acts on monoaminergic systems.In vitro,methylone has one third the potency of MDMA at inhibiting platelet serotonin accumulation and about the same in its inhibiting effects on the dopamine and noradrenaline transporters.[7][8][5]
In spite of these behavioral and pharmacological similarities between methylone and MDMA, the observed subjective effects of both drugs are not completely identical.Alexander Shulginwrote of the former:[9]
"[Methylone] has almost the same potency of MDMA, but it does not produce the same effects. It has an almost antidepressant action, pleasant and positive, but not the unique magic of MDMA."
In acute pharmacological studies of methylone (50–300mg) in humans, the drug produced physiological and psychological effects including increasedblood pressure,heart rate,body temperature,pupil dilation,stimulation,euphoria,feelings ofwell-being,enhancedempathy,increasedsociability,andaltered perception.[10][11]The studies found that the effects of methylone were similar to or milder than those of MDMA.[10][11]Methylone had a fasteronset of actionand its subjective effects wore off sooner than MDMA, which might lead to a redosing pattern of use.[10]Themisuse potentialof methylone, as measured by for instancedrug likingresponses, appeared to be similar to that of MDMA.[10]However it also has less off-target effects than MDMA which may be an advantage for medical applications.[12][13][14]
Pharmacology[edit]
Pharmacodynamics[edit]
Methylone acts as a mixedreuptake inhibitorandreleasing agentofserotonin,norepinephrine,anddopamine.[5][15]In comparison to MDMA, it has approximately 3x loweraffinityfor theserotonin transporter,while its affinity for thenorepinephrineanddopamine transportersis similar.[5][15]Notably, methylone's affinity for thevesicular monoamine transporter 2(VMAT2) is about 13x lower than that of MDMA.[5]The results of these differences in pharmacology relative to MDMA are that methylone is lesspotentin terms ofdose,has more balancedcatecholaminergiceffects relative toserotonergic,and behaves more like a reuptake inhibitor likemethylphenidatethan a releaser likeamphetamine;however, methylone has relatively robust releasing capabilities,[15]perhaps due to its ability tophosphorylatethemonoamine transportersbeing similar in potency relative to MDMA.[citation needed]
Pharmacokinetics[edit]
The two majormetabolic pathwaysinmammalsfor methylone areN-demethylationtomethylenedioxycathinone(MDC), anddemethylationfollowed byO-methylationof the 3- or 4-hydroxygroupto4-hydroxy-3-methoxymethcathinone(HMMC) or3-hydroxy-4-methoxymethcathinone(3-OH-4-MeO-MC). When 5 mg/kg of methylone was administered to rats, it was found that around 26% was excreted as HMMC within the first 48 hours (less than 3% excreted unchanged).[16]The meanelimination half-livesof methylone in humans followingoral administrationof doses of 50 to 200mg ranged from 5.8 to 6.9hours.[3]Theonset of actionandduration of actionof methylone in humans are 0.5hours and 2.5 to 3.0hours, respectively.[3]
Commercial distribution[edit]

Analysis of "Explosion" has confirmed that the active ingredient is methylone.[17][unreliable source?]Many other formulations marketed as household chemicals, as well as the pure powder, have been sold.
Legal status[edit]
Netherlands[edit]
In the Netherlands, methylone is not yet listed under theOpium Law,but is covered under the medicine act. Because methylone is not registered officially, it is forbidden to trade in methylone. The Minister of Health has asked the Coordination point Assessment and Monitoring new drugs group (CAM) to gather information about this substance, resulting possibly in an official risk assessment.[18]Until now, no research has been conducted on the toxicity of methylone, so nothing is known about the harmfulness of this new drug.
New Zealand[edit]
In New Zealand, although methylone is not explicitly scheduled and falls outside the strict definitions of an "amphetamine analogue" in the Misuse of Drugs Act, it is considered to be "substantially similar" to methcathinone and is thus considered by law enforcement authorities to be a Class C illegal drug. Methylone was sold in New Zealand for around 6 months from November 2005 to April 2006 as an MDMA substitute, under the name "Ease". The product was withdrawn after legal disputes with the government.[19][20]
UK[edit]
In the UK, methylone is illegal since the 16/04/2010 revision of the misuse of drugs act. Before this it was not specifically mentioned in United Kingdom (U.K.) law as the β-ketone was not covered under theMisuse of Drugs Act.In March 2010, plans were announced to make methylone and other cathinones, Class B drugs, "within weeks". While delayed by dissatisfaction in theAdvisory Council on the Misuse of Drugs,the revision was rushed through by the government with little regard for the views of the council. The importation of the compounds was banned immediately.[21]
Sweden[edit]
Sveriges riksdagadded methylone to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Oct 1, 2010, published byMedical Products Agencyin their regulation LVFS 2010:23 listed as Metylon, 2-metylamino-1-(3,4-metylendioxifenyl)propan-1-on.[22]Methylone was first classified bySveriges riksdagshealth ministryStatens folkhälsoinstitutas "health hazard" under the actLagen om förbud mot vissa hälsofarliga varor(translatedAct on the Prohibition of Certain Goods Dangerous to Health) as of Nov 1, 2005, in their regulation SFS 2005:733 listed as 3,4-metylendioximetkatinon (Metylon).[23]
Canada[edit]
Although not listed as aSchedule 1[24]substance, Health Canada reports that methylone falls under the scheduling as an analogue of amphetamine. However, Methylone bears the exact chemical difference between amphetamine andcathinone– and cathinone is listed as not being an analogue of amphetamine, possibly implying that methylone is unscheduled in Canada.[25]The CDSA was updated as a result of the Safe Streets Act changing amphetamines fromSchedule 3toSchedule 1;however, methylone was not added.[26]
United States[edit]
In October 2011, theDEAissued an emergency ban on methylone. It was made illegal to possess and distribute.[27][28]On April 4, 2013, the DEA placed methylone as a Schedule 1 substance under the CSA.[29]
- Arizona:
- Effective February 16, 2012, methylenedioxymethcathinone (methylone) was classified as a dangerous drug, making it a felony to knowingly possess, use, possess for sale, manufacture, administer, transport for sale, import into the state, or offer to transport for sale or import into this state, sell, transfer or offer to sell or transfer. A.R.S. 13-3401(6)(c)(xliii), 2012 Ariz. Legis. Serv. Ch. 1 (H.B. 2356).
- Florida:
- In January 2011, it was reported that Florida Attorney General Pam Bondi issued an emergency ban on MDPV, Methylone, Mephedrone, 3-methoxymethcathinone, 3-fluoromethcathinone, and 4-fluoromethcathinone as media attention on products labeled as "bath salts" grew. These chemicals are now Schedule I under Florida law.[30]
- Louisiana:
- In January 2011, Louisiana Governor Bobby Jindal emergency scheduled 3,4-methylenedioxymethcathinone (methylone), 3,4-methyenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone), 4-methoxymethcathinone (methedrone), 4-fluoromethcathinone (flephedrone), and 3-fluoromethcathinone (3-FMC).
- Tennessee:
- On May 5, 2011, Tennessee GovernorBill Haslamsigned a law making it a crime to knowingly produce, manufacture, distribute, sell, offer for sale or possess with intent produce, manufacture, distribute, sell, or offer for sale any product containing 3,4-methylenedioxymethcathinone (methylone), 3,4-methyenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone), 4-methoxymethcathinone (methedrone), 4-fluoromethcathinone (flephedrone), and 3-fluoromethcathinone (3-FMC).[31]
- Texas:
- In September 2011, Texas added 3,4-methylenedioxy-N-methylcathinone to the Penalty Group 2 listing of the Health and Safety Code. Possession of a substance in penalty group 2 is a minimum of a state jail felony.
- Michigan:
- Schedule 1 controlled substance in 2012.
Etymology[edit]
"Methylone" is also a trademarked brand name for an injectable form ofmethylprednisolone,a corticosteroid hormone used to treat arthritis and severe allergic reactions; hence, methylone may be confused with it. Aside from context, they can be distinguished by the fact that the name will usually be capitalized when referring to the prescription drug.
A proposed alternate name is βk-MDMA, or beta-keto-MDMA. While this nomenclature has not caught on because the name "methylone" became widely used before the conflicting Methylone trademark was noticed, the analogous names for related chemicalsβk-MDEAandβk-MBDBhave become the established names for those substances.
See also[edit]
References[edit]
- ^Anvisa(2023-07-24)."RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial"[Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese).Diário Oficial da União(published 2023-07-25).Archivedfrom the original on 2023-08-27.Retrieved2023-08-27.
- ^"Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )".Internetowy System Aktów Prawnych.Retrieved17 June2011.
- ^abcdePoyatos L, Lo Faro AF, Berardinelli D, Sprega G, Malaca S, Pichini S, et al. (November 2022)."Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans".International Journal of Molecular Sciences.23(23): 14636.doi:10.3390/ijms232314636.PMC9736016.PMID36498963.
- ^WO 9639133,Jacob III P,Shulgin AT,"Novel N-Substituted-2-Amino-3',4'-Methylene-dioxypropiophenones", published 1996-12-12, assigned toNeurobiological Technologies Inc.
- ^abcdeCozzi NV, Sievert MK, Shulgin AT, Jacob P, Ruoho AE (September 1999). "Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines".European Journal of Pharmacology.381(1): 63–69.doi:10.1016/S0014-2999(99)00538-5.PMID10528135.
- ^Dal Cason TA, Young R, Glennon RA (December 1997). "Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs".Pharmacology, Biochemistry, and Behavior.58(4). Elsevier BV: 1109–1116.doi:10.1016/s0091-3057(97)00323-7.PMID9408221.S2CID9704972.
- ^Cozzi NV, Sievert MK, Shulgin AT, Jacob III P, Ruoho AE (1998). "Methcathinone and 2 methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one (methylone) selectively inhibit plasma membrane catecholamine reuptake transporters".Soc. Neurosci. Abs.24(341.8).
- ^Cozzi NV, Shulgin AT, Ruoho AE (1998). "Methcathinone (MCAT) and 2-methylamino-1-(3,4 methylenedioxyphenyl)propan-1-one (MDMCAT) inhibit [3H]serotonin uptake into human platelets ".Amer. Chem. Soc. Div. Med. Chem. Abs.215(152).
- ^"Cathinone | Ask Dr. Shulgin Online".Archived fromthe originalon 2010-04-13.Retrieved2010-01-17.
- ^abcdPoyatos L, Pérez-Mañá C, Hladun O, Núñez-Montero M, de la Rosa G, Martín S, et al. (2023)."Pharmacological effects of methylone and MDMA in humans".Frontiers in Pharmacology.14:1122861.doi:10.3389/fphar.2023.1122861.PMC9981643.PMID36873994.
- ^abPoyatos L, Papaseit E, Olesti E, Pérez-Mañá C, Ventura M, Carbón X, et al. (August 2021)."A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure".Biology.10(8): 788.doi:10.3390/biology10080788.PMC8389614.PMID34440023.
- ^Warner-Schmidt J, Pittenger C, Stogniew M, Mandell B, Olmstead SJ, Kelmendi B (2022)."Methylone, a rapid acting entactogen with robust anxiolytic and antidepressant-like activity".Frontiers in Psychiatry.13:1041277.doi:10.3389/fpsyt.2022.1041277.PMC9873307.PMID36704743.
- ^Poyatos L, Pérez-Mañá C, Hladun O, Núñez-Montero M, de la Rosa G, Martín S, et al. (2023)."Pharmacological effects of methylone and MDMA in humans".Frontiers in Pharmacology.14:1122861.doi:10.3389/fphar.2023.1122861.PMC9981643.PMID36873994.
- ^Warner-Schmidt J, Stogniew M, Mandell B, Rowland RS, Schmidt EF, Kelmendi B (2024-02-07)."Methylone is a rapid-acting neuroplastogen with less off-target activity than MDMA".Frontiers in Neuroscience.18:1353131.doi:10.3389/fnins.2024.1353131.PMC10882719.PMID38389788.
- ^abcNagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain".European Journal of Pharmacology.559(2–3): 132–137.doi:10.1016/j.ejphar.2006.11.075.PMID17223101.
- ^Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, et al. (August 2006). "Metabolism of the recently encountered designer drug, methylone, in humans and rats".Xenobiotica; the Fate of Foreign Compounds in Biological Systems.36(8): 709–723.doi:10.1080/00498250600780191.PMID16891251.S2CID10875717.
- ^"Methylone sold under" Explosion "and" Inpact "brand names in the Netherlands and Japan".www.erowid.org.Apr 2005. Archived fromthe originalon 2009-03-04.
- ^van Amsterdam JG, Best W, Opperhuizen A, de Wolff FA (February 2004). "Evaluation of a procedure to assess the adverse effects of illicit drugs".Regulatory Toxicology and Pharmacology.39(1). Elsevier BV: 1–4.doi:10.1016/j.yrtph.2003.09.001.hdl:10029/12622.PMID14746774.
- ^"Party pill sparks official concern".One News.7 April 2006.Archivedfrom the original on 9 February 2012.Retrieved23 October2011.
- ^"EASE trial terminated after conflicting advice".scoop.co.nz.April 9, 2006. Archived fromthe originalon 2012-09-29.
- ^"Suspected mephedrone-type compound seized at airport".BBC News. 1 April 2010.Retrieved3 April2010.
- ^"Läkemedelsverkets föreskrifter - LVFS och HSLF-FS"(PDF).Läkemedelsverket - Swedish Medical Products Agency.Archived(PDF)from the original on 2011-02-16.Retrieved2010-10-07.
- ^"Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor;"(PDF).notisum.se(in Swedish).Archived(PDF)from the original on 2016-03-04.Retrieved2015-10-10.
- ^"Controlled Drugs and Substances Act: Legislative history · Schedule I · Section 19: Tramadol [Proposed]; Amphetamines".isomerdesign.com.Archivedfrom the original on 10 November 2013.Retrieved28 March2018.
- ^"Controlled Drugs and Substances Act: Definitions and Interpretations".isomerdesign.com.Archivedfrom the original on 10 November 2013.Retrieved28 March2018.
- ^"The Safe Streets and Communities Act Four Components Coming into Force".18 October 2012. Archived fromthe originalon 18 October 2012.Retrieved28 March2018.
- ^"Chemicals Used in 'Bath Salts' Now Under Federal Control and Regulation".USA Dept of Justice.Archivedfrom the original on 25 April 2014.Retrieved22 April2014.
- ^"Schedules of Controlled Substances: Placement of Methylone Into Schedule I".usdoj.gov.Archivedfrom the original on 17 April 2014.Retrieved22 April2014.
- ^"Schedules of Controlled Substances: Placement of Methylone Into Schedule I".federalregister.gov.2014-04-12.Archivedfrom the original on 15 December 2014.Retrieved22 April2014.
- ^"Florida Synthetic Drug Scheduling Actions - 2011-2014"(PDF).myfloridalegal.com.Archived(PDF)from the original on 2015-03-20.Retrieved2017-04-08.
- ^"Welcome to the Tennessee Secretary of State's Website – Tennessee Secretary of State"(PDF).state.tn.us.Archived(PDF)from the original on 1 September 2013.Retrieved28 March2018.
