Mitochondrial ROS
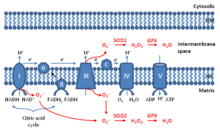
Mitochondrial ROS(mtROSormROS) arereactive oxygen species(ROS) that are produced bymitochondria.[1][2][3]Generation of mitochondrial ROS mainly takes place at theelectron transport chainlocated on theinner mitochondrial membraneduring the process ofoxidative phosphorylation.Leakage of electrons at complex I and complex III from electron transport chains leads to partial reduction of oxygen to formsuperoxide.Subsequently, superoxide is quickly dismutated tohydrogen peroxideby two dismutases includingsuperoxide dismutase 2(SOD2) inmitochondrial matrixand superoxide dismutase 1 (SOD1) in mitochondrial intermembrane space. Collectively, both superoxide and hydrogen peroxide generated in this process are considered as mitochondrial ROS.[1]
Once thought as merely the by-products of cellular metabolism, mitochondrial ROS are increasingly viewed as important signaling molecules,[4]whose levels of generation at 11 currently-identified sites vary depending on cellular energy supply and demand.[5][6]At low levels, mitochondrial ROS are considered to be important for metabolic adaptation as seen in hypoxia.[1]Mitochondrial ROS, stimulated by danger signals such aslysophosphatidylcholineandToll-like receptor 4andToll-like receptor 2bacterial ligandslipopolysaccharide(LPS) andlipopeptides,are involved in regulating inflammatory response.[7][8]Finally, high levels of mitochondrial ROS activateapoptosis/autophagypathways capable of inducing cell death.[9]
COVID-19
[edit]Monocytes/macrophages are the most enriched immune cell types in the lungs ofCOVID-19patients and appear to have a central role in the pathogenicity of the disease. These cells adapt their metabolism upon infection and become highly glycolytic, which facilitates SARS-CoV-2 replication. The infection triggers mitochondrial ROS production, which induces stabilization of hypoxia-inducible factor-1α (HIF1A) and consequently promotes glycolysis. HIF1A-induced changes in monocyte metabolism by SARS-CoV-2 infection directly inhibit T cell response and reduce epithelial cell survival. Targeting mitochondrial ROS may have great therapeutic potential for the development of novel drugs to treat patients with coronavirus.[10]
Aging
[edit]Mitochondrial ROS can promote cellularsenescenceandagingphenotypesin theskinof mice.[11]Ordinarily mitochondrialSOD2protects against mitochondrial ROS.Epidermalcells in mutant mice with a genetic SOD2 deficiency undergo cellular senescence, nuclearDNA damage,and irreversible arrest of proliferation in a portion of theirkeratinocytes.[11][12]
Mutant mice with a conditional deficiency for mitochondrial SOD2 inconnective tissuehave an accelerated agingphenotype.[13]This aging phenotype includes weight loss, skinatrophy,kyphosis(curvature of the spine),osteoporosis,muscle degenerationand reduced life span.
DNA damage
[edit]Mitochondrial ROS attackDNAreadily, generating a variety of DNA damages such asoxidized basesand strand breaks. The major mechanism that cells use to repair oxidized bases such as8-hydroxyguanine,formamidopyrimidineand5-hydroxyuracilisbase excision repair(BER).[14]BER occurs in both thecell nucleusand in mitochondria.
References
[edit]- ^abcLi X, Fang P, Mai J, et al. (February 2013)."Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers".J Hematol Oncol.6(19): 19.doi:10.1186/1756-8722-6-19.PMC3599349.PMID23442817.
- ^Reichart, Gesine (October 30, 2018). "Mitochondrial complex IV mutation increases ROS production and reduces lifespan in aged mice".Acta Physiologica.225(4): e13214.doi:10.1111/apha.13214.PMID30376218.S2CID53115753.
- ^Li X, Fang P, et al. (March 2017)."Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells".Can J Physiol Pharmacol.95(3): 247–252.doi:10.1139/cjpp-2016-0515.PMC5336492.PMID27925481.
- ^Trewin, Adam J; Bahr, Laura L; Almast, Anmol; Berry, Brandon J; Wei, Alicia Y; Foster, Thomas H; Wojtovich, Andrew P (2019-03-19)."Mitochondrial ROS generated at the complex-II matrix or intermembrane space microdomain have distinct effects on redox signaling and stress sensitivity in C. elegans".Antioxidants & Redox Signaling.31(9): 594–607.doi:10.1089/ars.2018.7681.ISSN1523-0864.PMC6657295.PMID30887829.
- ^Trewin, Adam J.; Parker, Lewan; Shaw, Christopher S.; Hiam, Danielle S.; Garnham, Andrew; Levinger, Itamar; McConell, Glenn K.; Stepto, Nigel K. (November 2018)."Acute HIIE elicits similar changes in human skeletal muscle mitochondrial H 2 O 2 release, respiration, and cell signaling as endurance exercise even with less work".American Journal of Physiology. Regulatory, Integrative and Comparative Physiology.315(5): R1003–R1016.doi:10.1152/ajpregu.00096.2018.hdl:10536/DRO/DU:30113706.ISSN0363-6119.PMID30183338.
- ^Goncalves, Renata L. S.; Quinlan, Casey L.; Perevoshchikova, Irina V.; Hey-Mogensen, Martin; Brand, Martin D. (2015-01-02)."Sites of Superoxide and Hydrogen Peroxide Production by Muscle Mitochondria Assessed ex Vivo under Conditions Mimicking Rest and Exercise".Journal of Biological Chemistry.290(1): 209–227.doi:10.1074/jbc.M114.619072.ISSN0021-9258.PMC4281723.PMID25389297.
- ^Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H, Yang X (April 2016)."Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation".Arteriosclerosis, Thrombosis, and Vascular Biology.36(6): 1090–100.doi:10.1161/ATVBAHA.115.306964.PMC4882253.PMID27127201.
- ^West AP (April 2011)."TLR signalling augments macrophage bactericidal activity through mitochondrial ROS".Nature.472(7344): 476–480.Bibcode:2011Natur.472..476W.doi:10.1038/nature09973.PMC3460538.PMID21525932.
- ^Finkel T (February 2012)."Signal transduction by mitochondrial oxidants".J Biol Chem.287(7): 4434–40.doi:10.1074/jbc.R111.271999.PMC3281633.PMID21832045.
- ^Cavounidis A, Mann EH (June 2020)."SARS-CoV-2 has a sweet tooth".Nature Reviews Immunology.20(8): 460.doi:10.2139/ssrn.3606770.PMC7291939.PMID32533110.
- ^abVelarde MC, Flynn JM, Day NU, Melov S, Campisi J (January 2012)."Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin".Aging (Albany NY).4(1): 3–12.doi:10.18632/aging.100423.PMC3292901.PMID22278880.
- ^Velarde MC, Demaria M, Melov S, Campisi J (August 2015)."Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells".Proc. Natl. Acad. Sci. U.S.A.112(33): 10407–12.Bibcode:2015PNAS..11210407V.doi:10.1073/pnas.1505675112.PMC4547253.PMID26240345.
- ^Treiber N, Maity P, Singh K, Kohn M, Keist AF, Ferchiu F, Sante L, Frese S, Bloch W, Kreppel F, Kochanek S, Sindrilaru A, Iben S, Högel J, Ohnmacht M, Claes LE, Ignatius A, Chung JH, Lee MJ, Kamenisch Y, Berneburg M, Nikolaus T, Braunstein K, Sperfeld AD, Ludolph AC, Briviba K, Wlaschek M, Florin L, Angel P, Scharffetter-Kochanek K (April 2011). "Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue".Aging Cell.10(2): 239–54.doi:10.1111/j.1474-9726.2010.00658.x.PMID21108731.
- ^Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA (January 2009)."Base excision repair of oxidative DNA damage and association with cancer and aging".Carcinogenesis.30(1): 2–10.doi:10.1093/carcin/bgn250.PMC2639036.PMID18978338.
