Myelin
| Myelin | |
|---|---|
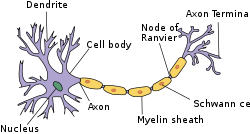 Structure of simplified neuron in thePNS | |
 Neuron with oligodendrocyte and myelin sheath in theCNS | |
| Details | |
| System | Nervous system |
| Identifiers | |
| FMA | 62977 |
| Anatomical terminology | |
Myelin(/ˈmaɪ.əlɪn/MY-ə-lin) is alipid-rich material that surroundsnervecellaxons(the nervous system's electrical wires) toinsulatethem and increase the rate at which electrical impulses (calledaction potentials) pass along the axon.[1][2]The myelinated axon can be likened to an electrical wire (the axon) with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin ensheaths the axon segmentally: in general, each axon is encased in multiple long sheaths with short gaps between, callednodes of Ranvier.At the nodes of Ranvier, which are approximately one thousandth of a mm in length, the axon's membrane is bare of myelin.
Myelin's best known function is to increase the rate at which information, encoded as electrical charges, passes along the axon's length. Myelin achieves this by elicitingsaltatory conduction.[1]Saltatory conduction refers to the fact that electrical impulses 'jump' along the axon, over long myelin sheaths, from one node of Ranvier to the next. Thus, information is passed around 100 times faster along a myelinated axon than a non-myelinated one.
At the molecular level, the myelin sheath increases the distance between extracellular and intracellular ions, reducing the accumulation of electrical charges. The discontinuous structure of the myelin sheath results in the action potential "jumping" from one node of Ranvier over a long (c. 0.1 mm – >1 mm, or 100-1000 micron) myelinated stretch of the axon called theinternodal segmentor "internode", before "recharging" at the next node of Ranvier. This 'jumping' continues until the action potential reaches theaxon terminal.[3][4][5]Once there, the electrical signal provokes the release of chemicalneurotransmittersacross thesynapse,which bind toreceptorson the post-synaptic cell (e.g. another neuron,myocyteorsecretory cell).
Myelin is made byglial cells,which are non-neuronal cells that providenutritionalandhomeostaticsupport to the axons. This is because axons, being elongated structures, are too far from thesomato be supported by the neurons themselves. In thecentral nervous system(brain,spinal cordandoptic nerves), myelination is formed by specialized glial cells calledoligodendrocytes,each of which sends out processes (limb-like extensions from the cell body) to myelinate multiple nearby axons; while in theperipheral nervous system,myelin is formed byneurolemmocytes(Schwann cells), which only myelinate a section of one axon. In the CNS, axons carry electrical signals from one nerve cell body to another.[6][7] The "insulating" function for myelin is essential for efficientmotor function(i.e. movement such as walking),sensory function(e.g.sight,hearing,smell,the feeling oftouchorpain) andcognition(e.g. acquiring and recalling knowledge), as demonstrated by the consequence of disorders that affect myelination, such as the genetically determinedleukodystrophies;[8]the acquired inflammatorydemyelinating disorder,multiple sclerosis;[9]and the inflammatory demyelinatingperipheral neuropathies.[10]Due to its high prevalence, multiple sclerosis, which specifically affects the central nervous system (brain, spinal cord and optic nerve), is the best known disorder of myelin.
Development[edit]
The process of generating myelin is calledmyelinationormyelinogenesis.In the CNS,oligodendrocyte progenitor cells(OPCs)differentiateinto mature oligodendrocytes, which form myelin. In humans, myelination begins early in the 3rd trimester,[11]although only little myelin is present in either the CNS or the PNS at the time of birth. During infancy, myelination progresses rapidly, with increasing numbers of axons acquiring myelin sheaths. This corresponds with the development of cognitive and motor skills, including language comprehension,speech acquisition,crawling and walking. Myelination continues through adolescence and early adulthood and although largely complete at this time, myelin sheaths can be added ingrey matterregions such as thecerebral cortex,throughout life.[12][13][14]
Species distribution[edit]
Vertebrates[edit]
Myelin is considered a defining characteristic of thejawedvertebrates(gnathostomes), though axons are ensheathed by a type of cell, called glial cells, in invertebrates.[15][16]These glial wraps are quite different from vertebrate compact myelin, formed, as indicated above, by concentric wrapping of the myelinating cell process multiple times around the axon. Myelin was first described in 1854 byRudolf Virchow,[17]although it was over a century later, following the development of electron microscopy, that its glial cell origin and its ultrastructure became apparent.[18]
In vertebrates, not all axons are myelinated. For example, in the PNS, a large proportion of axons are unmyelinated. Instead, they are ensheathed by non-myelinating Schwann cells known as Remak SCs and arranged inRemak bundles.[19]In the CNS, non-myelinated axons (or intermittently myelinated axons, meaning axons with long non-myelinated regions between myelinated segments) intermingle with myelinated ones and are entwined, at least partially, by the processes of another type ofglial celltheastrocyte.[citation needed][20]
Invertebrates[edit]
Functionally equivalent myelin-like sheaths are found in several invertebrate taxa, includingoligochaeteannelids, and crustacean taxa such aspenaeids,palaemonids,andcalanoids.These myelin-like sheaths share several structural features with the sheaths found in vertebrates including multiplicity of membranes, condensation of membrane, and nodes.[15]However, the nodes in vertebrates are annular; i.e. they encircle the axon. In contrast, nodes found in the sheaths of invertebrates are either annular or fenestrated; i.e. they are restricted to "spots". The fastest recorded conduction speed (across both vertebrates and invertebrates) is found in the ensheathed axons of theKuruma shrimp,an invertebrate,[15]ranging between 90 and 200 m/s[16](cf.100–120 m/s for the fastest myelinated vertebrate axon).
Composition[edit]


- Axon
- Nucleus of Schwann cell
- Schwann cell
- Myelin sheath
- Neurilemma
CNS myelin differs slightly in composition and configuration from PNS myelin, but both perform the same "insulating" function (see above). Being rich in lipid, myelin appears white, hence the name given to the "white matter"of the CNS. Both CNS white matter tracts (e.g. theoptic nerve,corticospinal tractandcorpus callosum) and PNS nerves (e.g. thesciatic nerveand theauditory nerve,which also appear white) each comprise thousands to millions of axons, largely aligned in parallel. Blood vessels provide the route for oxygen and energy substrates such as glucose to reach these fibre tracts, which also contain other cell types includingastrocytesandmicrogliain the CNS andmacrophagesin the PNS.
In terms of total mass, myelin comprises approximately 40% water; the dry mass comprises between 60% and 75%lipidand between 15% and 25%protein.Protein content includesmyelin basic protein(MBP),[21]which is abundant in the CNS where it plays a critical, non-redundant role in formation of compact myelin;myelin oligodendrocyte glycoprotein(MOG),[22]which is specific to the CNS; andproteolipid protein(PLP),[23]which is the most abundant protein in CNS myelin, but only a minor component of PNS myelin. In the PNS,myelin protein zero(MPZ or P0) has a similar role to that of PLP in the CNS in that it is involved in holding together the multiple concentric layers of glial cell membrane that constitute the myelin sheath. The primary lipid of myelin is aglycolipidcalledgalactocerebroside.The intertwining hydrocarbon chains ofsphingomyelinstrengthen the myelin sheath.Cholesterolis an essential lipid component of myelin, without which myelin fails to form.[24]
Function[edit]

The main purpose of myelin is to increase the speed at which electrical impulses (known asaction potentials) propagate along the myelinated fiber. In unmyelinated fibers, action potentials travel as continuous waves, but, in myelinated fibers, they "hop" or propagate bysaltatory conduction.The latter is markedly faster than the former, at least for axons over a certain diameter. Myelin decreasescapacitanceand increaseselectrical resistanceacross the axonal membrane (theaxolemma). It has been suggested that myelin permits larger body size by maintaining agile communication between distant body parts.[15]
Myelinated fibers lackvoltage-gated sodium channelsalong the myelinated internodes, exposing them only at thenodes of Ranvier.Here, they are highly abundant and densely packed.[25]Positively charged sodiumionscan enter the axon through these voltage-gated channels, leading todepolarisationof the membrane potential at the node of Ranvier. Theresting membrane potentialis then rapidly restored due to positively charged potassium ions leaving the axon throughpotassium channels.The sodium ions inside the axon then diffuse rapidly through the axoplasm (axonalcytoplasm), to the adjacent myelinated internode and ultimately to the next (distal) node of Ranvier, triggering the opening of the voltage gated sodium channels and entry of sodium ions at this site. Although the sodium ions diffuse through the axoplasm rapidly,diffusionis decremental by nature, thus nodes of Ranvier have to be (relatively) closely spaced, to secure action potential propagation.[26]The action potential "recharges" at consecutive nodes of Ranvier as the axolemmalmembrane potentialdepolarises to approximately +35 mV.[25]Along the myelinated internode, energy-dependent sodium/potassium pumps pump the sodium ions back out of the axon and potassium ions back into the axon to restore the balance of ions between the intracellular (inside the cell, i.e. axon in this case) and extracellular (outside the cell) fluids.
Whilst the role of myelin as an "axonal insulator" is well-established, other functions of myelinating cells are less well known or only recently established. The myelinating cell "sculpts" the underlying axon by promoting thephosphorylationofneurofilaments,thus increasing the diameter or thickness of the axon at the internodal regions; helps cluster molecules on the axolemma (such as voltage-gated sodium channels) at the node of Ranvier;[27]and modulates the transport ofcytoskeletalstructures andorganellessuch asmitochondria,along the axon.[28]In 2012, evidence came to light to support a role for the myelinating cell in "feeding" the axon.[29][30]In other words, the myelinating cell seems to act as a local "fueling station" for the axon, which uses a great deal of energy to restore the normal balance of ions between it and its environment,[31][32]following the generation of action potentials.
When a peripheral fiber is severed, the myelin sheath provides a track along which regrowth can occur. However, the myelin layer does not ensure a perfect regeneration of the nerve fiber. Some regenerated nerve fibers do not find the correct muscle fibers, and some damaged motor neurons of theperipheral nervous systemdie without regrowth. Damage to the myelin sheath and nerve fiber is often associated with increased functional insufficiency.
Unmyelinated fibers and myelinated axons of the mammalian central nervous system do not regenerate.[33]
Clinical significance[edit]
Demyelination[edit]
Demyelination is the loss of the myelin sheath insulating the nerves, and is the hallmark of someneurodegenerativeautoimmunediseases, includingmultiple sclerosis,acute disseminated encephalomyelitis,neuromyelitis optica,transverse myelitis,chronic inflammatory demyelinating polyneuropathy,Guillain–Barré syndrome,central pontine myelinosis,inherited demyelinating diseases such asleukodystrophy,andCharcot–Marie–Tooth disease.People withpernicious anaemiacan also develop nerve damage if the condition is not diagnosed quickly.Subacute combined degeneration of spinal cordsecondary to pernicious anaemia can lead to slight peripheral nerve damage to severe damage to the central nervous system, affecting speech, balance, andcognitiveawareness. When myelin degrades, conduction of signals along the nerve can be impaired or lost, and the nerve eventually withers.[clarification needed]A more serious case of myelin deterioration is calledCanavan disease.
Theimmune systemmay play a role in demyelination associated with such diseases, including inflammation causing demyelination by overproduction ofcytokinesvia upregulation oftumor necrosis factor[34]orinterferon.MRI evidence that docosahexaenoic acidDHAethyl ester improves myelination in generalized peroxisomal disorders.[35]
Symptoms[edit]
Demyelinationresults in diverse symptoms determined by the functions of the affected neurons. It disrupts signals between the brain and other parts of the body; symptoms differ from patient to patient, and have different presentations upon clinical observation and in laboratory studies.
Typical symptoms include blurriness in the central visual field that affects only one eye, may be accompanied by pain upon eye movement, double vision, loss of vision/hearing, odd sensation in legs, arms, chest, or face, such as tingling or numbness (neuropathy), weakness of arms or legs, cognitive disruption, including speech impairment and memory loss, heat sensitivity (symptoms worsen or reappear upon exposure to heat, such as a hot shower), loss of dexterity, difficulty coordinating movement or balance disorder, difficulty controlling bowel movements or urination, fatigue, and tinnitus.[36]
Myelin repair[edit]
Research to repair damaged myelin sheaths is ongoing. Techniques include surgically implantingoligodendrocyte precursor cellsin the central nervous system and inducingmyelin repairwith certainantibodies.While results in mice have been encouraging (viastem celltransplantation), whether this technique can be effective in replacing myelin loss in humans is still unknown.[37]Cholinergic treatments,such asacetylcholinesterase inhibitors(AChEIs), may have beneficial effects on myelination, myelin repair, and myelin integrity. Increasing cholinergic stimulation also may act through subtle trophic effects on brain developmental processes and particularly on oligodendrocytes and the lifelong myelination process they support. Increasingoligodendrocytecholinergic stimulation,AChEIs,and other cholinergic treatments, such asnicotine,possibly could promote myelination during development and myelin repair in older age.[38]Glycogen synthase kinase 3βinhibitors such aslithium chloridehave been found to promote myelination in mice with damaged facial nerves.[39]Cholesterol is a necessary nutrient for the myelin sheath, along withvitamin B12.[40][41]
Dysmyelination[edit]
Dysmyelination is characterized by a defective structure and function of myelin sheaths; unlike demyelination, it does not producelesions.Such defective sheaths often arise from genetic mutations affecting the biosynthesis and formation of myelin. Theshiverer mouserepresents one animal model of dysmyelination. Human diseases where dysmyelination has been implicated includeleukodystrophies(Pelizaeus–Merzbacher disease,Canavan disease,phenylketonuria) andschizophrenia.[42][43][44]
See also[edit]
- Lesional demyelinations of the central nervous system
- Myelin-associated glycoprotein
- Myelin incisure
- The Myelin Project,project to regenerate myelin
- Myelin Repair Foundation,a nonprofit medical research foundation formultiple sclerosisdrug discovery.
- Myelinoid,anin vitromodel for studying human myelination and white matter diseases
References[edit]
- ^abBean, Bruce P. (June 2007). "The action potential in mammalian central neurons".Nature Reviews Neuroscience.8(6): 451–465.doi:10.1038/nrn2148.ISSN1471-0048.PMID17514198.S2CID205503852.
- ^Morell, Pierre; Quarles, Richard H. (1999)."The Myelin Sheath".Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition.Lippincott-Raven.Retrieved15 December2023.
- ^Carroll, SL (2017)."The Molecular and Morphologic Structures That Make Saltatory Conduction Possible in Peripheral Nerve".Journal of Neuropathology and Experimental Neurology.76(4): 255–257.doi:10.1093/jnen/nlx013.PMID28340093.
- ^Keizer J, Smith GD, Ponce-Dawson S, Pearson JE (August 1998)."Saltatory propagation of Ca2+ waves by Ca2+ sparks".Biophysical Journal.75(2): 595–600.Bibcode:1998BpJ....75..595K.doi:10.1016/S0006-3495(98)77550-2.PMC1299735.PMID9675162.
- ^Dawson SP, Keizer J, Pearson JE (May 1999)."Fire-diffuse-fire model of dynamics of intracellular calcium waves".Proceedings of the National Academy of Sciences of the United States of America.96(11): 6060–3.Bibcode:1999PNAS...96.6060D.doi:10.1073/pnas.96.11.6060.PMC26835.PMID10339541.
- ^Stassart, Ruth M.; Möbius, Wiebke; Nave, Klaus-Armin; Edgar, Julia M. (2018)."The Axon-Myelin Unit in Development and Degenerative Disease".Frontiers in Neuroscience.12:467.doi:10.3389/fnins.2018.00467.ISSN1662-4548.PMC6050401.PMID30050403.
- ^Stadelmann, Christine; Timmler, Sebastian; Barrantes-Freer, Alonso; Simons, Mikael (2019-07-01)."Myelin in the Central Nervous System: Structure, Function, and Pathology".Physiological Reviews.99(3): 1381–1431.doi:10.1152/physrev.00031.2018.ISSN1522-1210.PMID31066630.
- ^van der Knaap MS, Bugiani M (September 2017)."Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms".Acta Neuropathologica.134(3): 351–382.doi:10.1007/s00401-017-1739-1.PMC5563342.PMID28638987.
- ^Compston A, Coles A (October 2008). "Multiple sclerosis".Lancet.372(9648): 1502–17.doi:10.1016/S0140-6736(08)61620-7.PMID18970977.S2CID195686659.
- ^Lewis RA (October 2017). "Chronic inflammatory demyelinating polyneuropathy".Current Opinion in Neurology.30(5): 508–512.doi:10.1097/WCO.0000000000000481.PMID28763304.S2CID4961339.
- ^"Pediatric Neurologic Examination Videos & Descriptions: Developmental Anatomy".library.med.utah.edu.Retrieved2016-08-20.
- ^Swire M, Ffrench-Constant C (May 2018)."Seeing Is Believing: Myelin Dynamics in the Adult CNS".Neuron.98(4): 684–686.doi:10.1016/j.neuron.2018.05.005.PMID29772200.
- ^Hill RA, Li AM, Grutzendler J (May 2018)."Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain".Nature Neuroscience.21(5): 683–695.doi:10.1038/s41593-018-0120-6.PMC5920745.PMID29556031.
- ^Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE (May 2018)."Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex".Nature Neuroscience.21(5): 696–706.doi:10.1038/s41593-018-0121-5.PMC5920726.PMID29556025.
- ^abcdHartline DK (May 2008). "What is myelin?".Neuron Glia Biology.4(2): 153–63.doi:10.1017/S1740925X09990263.PMID19737435.S2CID33164806.
- ^abSalzer JL, Zalc B (October 2016)."Myelination"(PDF).Current Biology.26(20): R971–R975.Bibcode:2016CBio...26.R971S.doi:10.1016/j.cub.2016.07.074.PMID27780071.
- ^Virchow R (1854)."Ueber das ausgebreitete Vorkommen einer dem Nervenmark analogen Substanz in den thierischen Geweben".Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin(in German).6(4): 562–572.doi:10.1007/BF02116709.S2CID20120269.
- ^Boullerne AI (September 2016)."The history of myelin".Experimental Neurology.283(Pt B): 431–445.doi:10.1016/j.expneurol.2016.06.005.PMC5010938.PMID27288241.
- ^Monk KR, Feltri ML, Taveggia C (August 2015)."New insights on Schwann cell development".Glia.63(8): 1376–1393.doi:10.1002/glia.22852.PMC4470834.PMID25921593.
- ^Wang, Doris D.; Bordey, Angélique (11 December 2008)."The astrocyte odyssey".Progress in Neurobiology.86(4): 342–367.doi:10.1016/j.pneurobio.2008.09.015.PMC2613184.PMID18948166– via Elsevier Science Direct.
- ^Steinman L (May 1996)."Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system".Cell.85(3): 299–302.doi:10.1016/S0092-8674(00)81107-1.PMID8616884.S2CID18442078.
- ^Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S (April 2015)."The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis".Progress in Neurobiology.127–128: 1–22.doi:10.1016/j.pneurobio.2015.02.003.PMC4578232.PMID25802011.
- ^Greer JM, Lees MB (March 2002). "Myelin proteolipid protein--the first 50 years".The International Journal of Biochemistry & Cell Biology.34(3): 211–215.doi:10.1016/S1357-2725(01)00136-4.PMID11849988.
- ^Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA (April 2005). "High cholesterol level is essential for myelin membrane growth".Nature Neuroscience.8(4): 468–475.doi:10.1038/nn1426.PMID15793579.S2CID9762771.
- ^abSaladin KS (2012).Anatomy & physiology: the unity of form and function(6th ed.). New York, NY: McGraw-Hill.[page needed]
- ^Raine CS (1999)."Characteristics of Neuroglia".In Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD (eds.).Basic Neurochemistry: Molecular, Cellular and Medical Aspects(6th ed.). Philadelphia: Lippincott-Raven.
- ^Brivio V, Faivre-Sarrailh C, Peles E, Sherman DL, Brophy PJ (April 2017)."Assembly of CNS Nodes of Ranvier in Myelinated Nerves Is Promoted by the Axon Cytoskeleton".Current Biology.27(7): 1068–1073.Bibcode:2017CBio...27.1068B.doi:10.1016/j.cub.2017.01.025.PMC5387178.PMID28318976.
- ^Stassart RM, Möbius W, Nave KA, Edgar JM (2018)."The Axon-Myelin Unit in Development and Degenerative Disease".Frontiers in Neuroscience.12:467.doi:10.3389/fnins.2018.00467.PMC6050401.PMID30050403.
- ^Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (April 2012)."Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity".Nature.485(7399): 517–521.Bibcode:2012Natur.485..517F.doi:10.1038/nature11007.PMC3613737.PMID22622581.
- ^Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (July 2012)."Oligodendroglia metabolically support axons and contribute to neurodegeneration".Nature.487(7408): 443–448.Bibcode:2012Natur.487..443L.doi:10.1038/nature11314.PMC3408792.PMID22801498.
- ^Engl E, Attwell D (August 2015)."Non-signalling energy use in the brain".The Journal of Physiology.593(16): 3417–329.doi:10.1113/jphysiol.2014.282517.PMC4560575.PMID25639777.
- ^Attwell D, Laughlin SB (October 2001)."An energy budget for signaling in the grey matter of the brain".Journal of Cerebral Blood Flow and Metabolism.21(10): 1133–145.doi:10.1097/00004647-200110000-00001.PMID11598490.
- ^Huebner, Eric A.; Strittmatter, Stephen M. (2009)."Axon Regeneration in the Peripheral and Central Nervous Systems".Results and Problems in Cell Differentiation.48:339–351.doi:10.1007/400_2009_19.ISBN978-3-642-03018-5.ISSN0080-1844.PMC2846285.PMID19582408.
- ^Ledeen RW, Chakraborty G (March 1998). "Cytokines, signal transduction, and inflammatory demyelination: review and hypothesis".Neurochemical Research.23(3): 277–289.doi:10.1023/A:1022493013904.PMID9482240.S2CID7499162.
- ^Martinez, Manuela; Vazquez, Elida (1 July 1998)."MRI evidence that docosahexaenoic acid ethyl ester improves myelination in generalized peroxisomal disorders".Neurology.51(1): 26–32.doi:10.1212/wnl.51.1.26.PMID9674774.S2CID21929640.
- ^Mayo Clinic 2007 and University of Leicester Clinical Studies, 2014[full citation needed]
- ^Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA (January 2004). "Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain".Nature Medicine.10(1): 93–97.doi:10.1038/nm974.PMID14702638.S2CID34822879.
- "Stem Cell Therapy Replaces Missing Myelin In Mouse Brains".FuturePundit.January 20, 2004. Archived fromthe originalon June 14, 2011.RetrievedMarch 22,2007.
- ^Bartzokis G (August 2007). "Acetylcholinesterase inhibitors may improve myelin integrity".Biological Psychiatry.62(4): 294–301.doi:10.1016/j.biopsych.2006.08.020.PMID17070782.S2CID2130691.
- ^Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G, Fledrich R, Fonte C, Branchu J, Goulard M, de Waele C, Charbonnier F, Sereda MW, Baulieu EE, Schumacher M, Bernard S, Massaad C (March 2012)."Lithium enhances remyelination of peripheral nerves".Proceedings of the National Academy of Sciences of the United States of America.109(10): 3973–3978.Bibcode:2012PNAS..109.3973M.doi:10.1073/pnas.1121367109.PMC3309729.PMID22355115.
- ^Petrov AM, Kasimov MR, Zefirov AL (2016)."Brain Cholesterol Metabolism and Its Defects: Linkage to Neurodegenerative Diseases and Synaptic Dysfunction".Acta Naturae.8(1): 58–73.doi:10.32607/20758251-2016-8-1-58-73.PMC4837572.PMID27099785.
- ^Miller A, Korem M, Almog R, Galboiz Y (June 2005). "Vitamin B12, demyelination, remyelination and repair in multiple sclerosis".Journal of the Neurological Sciences.233(1–2): 93–97.doi:10.1016/j.jns.2005.03.009.PMID15896807.S2CID6269094.
- ^Krämer-Albers EM, Gehrig-Burger K, Thiele C, Trotter J, Nave KA (November 2006)."Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia".The Journal of Neuroscience.26(45): 11743–1752.doi:10.1523/JNEUROSCI.3581-06.2006.PMC6674790.PMID17093095.
- ^Matalon R, Michals-Matalon K, Surendran S, Tyring SK (2006). "Canavan disease: studies on the knockout mouse".N-Acetylaspartate.Advances in Experimental Medicine and Biology. Vol. 576. pp. 77–93, discussion 361–363.doi:10.1007/0-387-30172-0_6.ISBN978-0-387-30171-6.PMID16802706.
- ^Tkachev D, Mimmack ML, Huffaker SJ, Ryan M, Bahn S (August 2007)."Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia".The International Journal of Neuropsychopharmacology.10(4): 557–563.doi:10.1017/S1461145706007334.PMID17291371.
Further reading[edit]
- Fields, R. Douglas, "The Brain Learns in Unexpected Ways: Neuroscientists have discovered a set of unfamiliar cellular mechanisms for making fresh memories",Scientific American,vol. 322, no. 3 (March 2020), pp. 74–79. "Myelin, long considered inert insulation onaxons,is now seen as making a contribution to learning by controlling the speed at which signals travel along neural wiring. "(p. 79.)
- Swire M, Ffrench-Constant C (May 2018)."Seeing Is Believing: Myelin Dynamics in the Adult CNS".Neuron.98(4): 684–686.doi:10.1016/j.neuron.2018.05.005.PMID29772200.
- Waxman SG (October 1977). "Conduction in myelinated, unmyelinated, and demyelinated fibers".Archives of Neurology.34(10): 585–9.doi:10.1001/archneur.1977.00500220019003.PMID907529.
