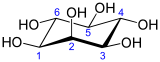
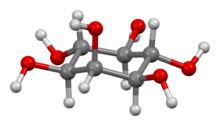
Inositol

| |
| |
| |
| Names | |
|---|---|
| IUPAC name
myo-Inositol
| |
| Systematic IUPAC name
(1R,2S,3r,4R,5S,6s)-Cyclohexane-1,2,3,4,5,6-hexol | |
| Other names
cis-1,2,3,5-trans-4,6-Cyclohexanehexol
Cyclohexanehexol Mouse antialopecia factor Nucite Phaseomannite Phaseomannitol Rat antispectacled eye factor Scyllite(for the isomerscyllo-inositol) Vitamin B8 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.295 |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
| Density | 1.752 g/cm3 |
| Melting point | 225 to 227 °C (437 to 441 °F; 498 to 500 K) |
| Thermochemistry[2] | |
Std enthalpy of
formation(ΔfH⦵298) |
−1329.3 kJ/mol |
Std enthalpy of
combustion(ΔcH⦵298) |
−2747 kJ/mol |
| Pharmacology | |
| A11HA07(WHO) | |
| Hazards | |
| NFPA 704(fire diamond) | |
| Flash point | 143 °C (289 °F; 416 K) |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Inbiochemistry,medicine,and related sciences,inositolgenerally refers tomyo-inositol(formerlymeso-inositol), the most importantstereoisomerof thechemical compoundcyclohexane-1,2,3,4,5,6-hexol.ItsformulaisC6H12O6;the molecule has a ring of sixcarbonatoms, each with anhydrogenatom and ahydroxylgroup (–OH). Inmyo-inositol, two of the hydroxyls, neither adjacent not opposite, lie above the respective hydrogens relative to the mean plane of the ring.
The compound is acarbohydrate,specifically asugar alcohol(as distinct fromaldoseslikeglucose) with half thesweetnessofsucrose(table sugar). It is one of the most ancient components of living beings with multiple functions in eukaryotes, including structural lipids and secondary messengers.[3]A human kidney makes about two grams per day fromglucose,but other tissues synthesize it too. The highest concentration is in the brain, where it plays an important role in making otherneurotransmittersand somesteroid hormonesbind to their receptors.[4]In other tissues, it mediates cell signal transduction in response to a variety ofhormones,neurotransmitters,and growth factors and participates inosmoregulation.[5]In most mammalian cells the concentrations ofmyo-inositol are 5 to 500 times greater inside cells than outside them.[6]
A 2023 meta-analysis found that inositol is a safe and effective treatment in the management of polycystic ovary syndrome (PCOS).[7]However, there is only evidence of very low quality for its efficacy in increasing fertility for IVF in women with PCOS.[8]
The other naturally occurring stereoisomers of cyclohexane-1,2,3,4,5,6-hexol arescyllo-,muco-,D-chiro-,L-chiro-, andneo-inositol,although they occur in minimal quantities compared tomyo-inositol. The other possible isomers areallo-,epi-, andcis-inositol.
History
[edit]myo-Inositol was originally isolated from muscle extracts by Johanes Joseph Scherer (1814–1869) in 1850.[3]It was formerly calledmeso-inositol to distinguish it from thechiro- isomer. However, since all other isomers are meso (non-chiral) compounds, the namemyo-inositol is now preferred (myo- being amedical prefixfor "muscle" ).
Inositol was once considered a member of thevitamin Bcomplex, namelyvitamin B8before the discovery that it is made naturally in the human body, and therefore cannot be avitaminoressential nutrient.[9]
Chemical properties
[edit]myo-Inositol is ameso compound,meaning it isoptically inactivebecause it has aplane of symmetry.[10]It is a white crystalline powder, relatively stable in the air. It is highly soluble in water, slightly soluble inglacial acetic acid,ethanol,glycol,andglycerin,but insoluble inchloroformandether.[3]
In its most stableconformation,themyo-inositol isomer assumes thechair conformation,which moves the maximum number of hydroxyls to the equatorial position, where they are farthest apart from each other. In this conformation, the naturalmyoisomer has a structure in which five of the sixhydroxyls(the first, third, fourth, fifth, and sixth) areequatorial,whereas the second hydroxyl group isaxial.[11]
Physiological roles
[edit]myo-Inositol plays an important role as the structural basis for a number ofsecondary messengersineukaryoticcells,the variousinositol phosphates.In addition, inositol serves as an important component of the structural lipidsphosphatidylinositol(PI) and its various phosphates, thephosphatidylinositol phosphate(PIP) lipids.
Biosynthesis
[edit]In humans,myo-Inositol issynthesizedde novobutD-chiro-inositol is not.[6]myo-Inositol is synthesized fromglucose 6-phosphate(G6P) in two steps. First, G6P isisomerisedby aninositol-3-phosphate synthaseenzyme (for example,ISYNA1) tomyo-inositol 1-phosphate, which is then dephosphorylated by aninositol monophosphataseenzyme (for example,IMPA1) to give freemyo-inositol. In humans, most inositol is synthesized in the kidneys, followed by testicles, typically in amounts of a few grams per day.[5]
At the peripheral level,myo-inositol is converted toD-chiro-inositol by a specific epimerase. Only a minor fraction ofmyo-inositol is converted intoD-chiro-inositol.[6]The activity of this epimerase is insulin dependent, causing a reduction ofD-chiro-inositol in muscle, fat, and liver when there isinsulin resistance.[12][6]D-chiro-inositol reduces the conversion of testosterone to estrogen, thereby increases the levels of testosterone and worsening PCOS.[6]
Phytic acid in plants
[edit]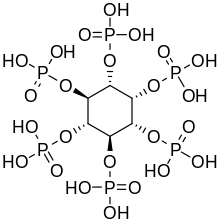
Inositol hexaphosphate, also calledphytic acidor IP6, is aphytochemicaland the principal storage form ofphosphorusin manyplanttissues,especiallybranandseed.[13]Phosphorus and inositol in phytate form are not generallybioavailableto non-ruminantanimals because these animals lack the digestiveenzymephytaserequired to remove the phosphate groups. Ruminants are readily able to digest phytate because of the phytase produced by microorganisms in therumen.[14]Moreover, phytic acid alsochelatesimportant minerals such ascalcium,magnesium,iron,andzinc,making them unabsorbable, and contributing to mineral deficiencies in people whose diets rely highly on bran and seeds for their mineral intake, such as occurs indeveloping countries.[15][16]
Inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3) are also called "phytates".
Inositol or its phosphates and associated lipids are found in many foods, in particular fruit, especiallycantaloupeandoranges.[17]In plants, the hexaphosphate of inositol,phytic acidor its salts, the phytates, serve as phosphate stores in seed, for example in nuts and beans.[18]Phytic acid also occurs incerealswith highbrancontent. Phytate is, however, not directlybioavailableto humans in the diet, since it is not digestible. Some food preparation techniques partly break down phytates to change this. However, inositol in the form ofphospholipids,as found in certain plant-derived substances such aslecithins,is well absorbed and relatively bioavailable.
Biological function
[edit]Inositol, phosphatidylinositol and some of their mono- and polyphosphates function assecondary messengersin a number of intracellularsignal transductionpathways. They are involved in a number of biological processes, including:
- Insulinsignal transduction[19]
- Cytoskeletonassembly
- Nerve guidance (epsin)
- Intracellularcalcium(Ca2+) concentration control[20]
- Cellmembrane potentialmaintenance[21]
- Breakdown offats[22]
- Gene expression[23][24]
In one important family of pathways,phosphatidylinositol 4,5-bisphosphate(PIP2) is stored in cellular membranes until it is released by any of a number of signalling proteins and transformed into various secondary messengers, for examplediacylglycerolandinositol triphosphate.[25]
'myo-Inositol has very low toxicity, with a reportedLD5010,000 mg/kg body weight (oral) in rats.[3]
Industrial uses
[edit]Explosives industry
[edit]At the 1936 meeting of theAmerican Chemical Society,professorEdward Bartowof theUniversity of Iowapresented a commercially viable means of extracting large amounts of inositol from the phytic acid naturally present in waste corn. As a possible use for the chemical, he suggested 'inositol nitrate' as a more stable alternative tonitroglycerin.[26]Today, inositol nitrate is used to gelatinizenitrocellulosein many modern explosives and solid rocket propellants.[27]
Road salt
[edit]When plants are exposed to increasing concentrations ofroad salt,the plant cells become dysfunctional and undergoapoptosis,leading to inhibited growth. Inositol pretreatment could reverse these effects.[28]
Research and clinical applications
[edit]Trichotillomania
[edit]High doses of inositol may be used to treattrichotillomania(compulsive hair-pulling) and related disorders.[29]
Other illnesses
[edit]D-chiro-inositol is an important messenger molecule in insulin signaling.[30]Inositol supplementation has been shown to significantly decreasetriglyceridesandLDL cholesterolin patients withmetabolic diseases.[30]
myo-Inositol is important forthyroid hormonesynthesis.[31]Depletion ofmyo-inositol may predispose to development ofhypothyroidism.[31]Patients with hypothyroidism have a higher demand formyo-inositol than healthy subjects.[31]
Inositol should not be routinely implemented for the management of preterm babies who have or are at a risk ofinfant respiratory distress syndrome(RDS).[32]Myo-inositol helps preventneural tube defectswith particular efficacy in combination withfolic acid.[33]
Inositol is considered a safe and effective treatment forpolycystic ovary syndrome(PCOS).[7]It works by increasing insulin sensitivity, which helps to improve ovarian function and reducehyperandrogenism.[34]It is also shown to reduce the risk ofmetabolic diseasein women with PCOS.[35]In addition, thanks to its role as FSH second messenger,myo-inositol is effective in restoring FSH/LH ratio and menstrual cycle regularization.[36]myo-Inositol's role as FSH second messenger leads to a correct ovarian follicle maturation and consequently to a higher oocyte quality. Improving the oocyte quality in both women with or without PCOS,myo-inositol can be considered as a possible approach for increasing the chance of success in assisted reproductive technologies.[37][38]In contrast,D-chiro-inositol can impair oocyte quality in a dose-dependent manner.[39]The high level of DCI seems to be related to elevated insulin levels retrieved in about 70% of PCOS women.[40]In this regard, insulin stimulates the irreversible conversion ofmyo-inositol toD-chiro-inositol causing a drastic reduction ofmyo-inositol.myo-Inositol depletion is particularly damaging to ovarian follicles because it is involved in FSH signaling, which is impaired due tomyo-inositol depletion.[12]Recent evidence reports a faster improvement of the metabolic and hormonal parameters when these two isomers are administered in their physiological ratio. The plasmatic ratio ofmyo-inositol andD-chiro-inositol in healthy subjects is 40:1 ofmyo- andD-chiro-inositol respectively.[41]The use of the 40:1 ratio shows the same efficacy ofmyo-inositol alone but in a shorter time. In addition, the physiological ratio does not impair oocyte quality.[42]
The use of inositols in PCOS is gaining more importance, and an efficacy higher than 70% with a strong safety profile is reported. On the other hand, about 30% of patients could show as inositol-resistant.[43]New evidence regarding PCOS aetiopathogenesis describes an alteration in the species and the quantity of each strain characterizing the normal gastrointestinal flora. This alteration could lead to chronic, low-level inflammation and malabsorption.[44]A possible solution could be represented by the combination ofmyo-inositol andα-lactalbumin.This combination shows a synergic effect in increasing myo-inositol absorption.[45]A recent study reported that themyo-inositol and α-lactalbumin combination is able to increasemyo-inositol plasmatic content in inositol-resistant patients with a relative improvement of hormonal and metabolic parameters.[46]
Use as a cutting agent
[edit]Inositol has been used as an adulterant orcutting agentfor many illegal drugs, such ascocaine,methamphetamine,and sometimesheroin,[47]probably because of its solubility, powdery texture, or reducedsweetness(50%) compared to more common sugars.
Inositol is also used as a stand-infilm propforcocaineinfilmmaking.[48][49]
Nutritional sources
[edit]myo-Inositol is naturally present in a variety of foods, although tables of food composition do not always distinguish betweenlecithin,the relatively bioavailable lipid form and the biounavailable phytate/phosphate form.[17]Foods containing the highest concentrations ofmyo-inositol and its compounds include fruits, beans, grains, and nuts.[17]Fruits in particular, especially oranges and cantaloupe, contain the highest amounts ofmyo-inositol.[50]It is also present in beans, nuts, and grains, however, these contain large amounts ofmyo-inositol in the phytate form, which is not bioavailable without transformation byphytaseenzymes.Bacillus subtilis,the microorganism which produces the fermented foodnatto,produces phytase enzymes that may convertphytic acidto a more bioavailable form of inositol polyphosphate in the gut.[51]Additionally,Bacteroidesspecies in the gut secrete vesicles containing an active enzyme which converts the phytate molecule into bioavailable phosphorus and inositol polyphosphate, which is an important signaling molecule in the human body.[52]
myo-Inositol can also be found as an ingredient inenergy drinks,[53]either in conjunction with or as a substitute for glucose.[54]
In humans, myo-inositol is naturally made from glucose-6-phosphate through enzymatic dephosphorylation.[50]
Production
[edit]As of 2021, the main industrial process for the production ofmyo-inositol (mostly in China and Japan) started with phytate (IP6) extracted from the soaking water resulting from corn and rice bran processing. After purification, the phytate is hydrolized, andmyo-inositol is separated by crystallization.[3]
Another route is microbial fermentation of carbohydrates by various organisms, such as the fungusNeurospora crassa(Beadle and Tatum, 1945),Candida boidini(Shirai et al., 1997),Saccharomyces cerevisiae(Culbertson et al., 1976),Escherichia coli(Hansen, 1999).[3]Alternatively, enzime extracts from microbial cultures can be used in vitro to obtainmyo-inositol from various substrates, including glucose,sucrose,starch,xylose,andamylose.[3]
References
[edit]- ^Merck Index(11th ed.). p. 4883.
- ^Knyazev, A.V.; Emel’yanenko, V.N.; Shipilova, A.S.; Zaitsau, D.H.; Lelet, M.I.; Knyazeva, S.S.; Gusarova, E.V.; Varfolomeev, M.A. (2018)."Thermodynamic properties of myo-inositol".The Journal of Chemical Thermodynamics.116:76–84.doi:10.1016/j.jct.2017.08.028.ISSN0021-9614.
- ^abcdefgYunjie Li, Pingping Han, Juan Wang, Ting Shi, Chun You (2021): "Production of myo-inositol: Recent advance and prospective".Biotechnology and Applied Biochemistry,volume 69, issue 3, pages 1101-1111.doi:10.1002/bab.2181
- ^Croze, M. L.; Soulage, C. O. (October 2013). "Potential role and therapeutic interests ofmyo-inositol in metabolic diseases ".Biochimie.95(10): 1811–1827.doi:10.1016/j.biochi.2013.05.011.PMID23764390.
- ^abParthasarathy, L. K.; Seelan, R. S.; Tobias, C.; Casanova, M. F.; Parthasarathy, R. N. (2006).Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent.Subcellular Biochemistry. Vol. 39. pp. 293–314.doi:10.1007/0-387-27600-9_12.ISBN978-0-387-27599-4.PMID17121280.
- ^abcdeKiani AK, Paolacci S, Bertelli M (2021). "From Myo-inositol to D-chiro-inositol molecular pathways".European Review for Medical and Pharmacological Sciences.25(5): 2390–2402.doi:10.26355/eurrev_202103_25279.PMID33755975.
- ^abGreff, Dorina; Juhász, Anna E.; Váncsa, Szilárd; Váradi, Alex; Sipos, Zoltán; Szinte, Julia; Park, Sunjune; Hegyi, Péter; Nyirády, Péter; Ács, Nándor; Várbíró, Szabolcs; Horváth, Eszter M. (2023)."Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials".Reproductive Biology and Endocrinology.21(1): 10.doi:10.1186/s12958-023-01055-z.PMC9878965.PMID36703143.
- ^Showell, M. G.; Mackenzie-Proctor, R.; Jordan, V.; Hodgson, R.; Farquhar, C. (2018)."Inositol for subfertile women with polycystic ovary syndrome".The Cochrane Database of Systematic Reviews.2018(12): CD012378.doi:10.1002/14651858.CD012378.pub2.PMC6516980.PMID30570133.
- ^J. E. F. Reynolds (1993):Martindale: The Extra Pharmacopoeia,volume 30. Quote (page 1379): "An isomer of glucose that has traditionally been considered to be a B vitamin although it has an uncertain status as a vitamin and a deficiency syndrome has not been identified in man".ISBN978-0-85369-300-0
- ^Majumder, A. L.; Biswas, B. B. (2006-10-03).Biology of Inositols and Phosphoinositides.Springer Science & Business Media.ISBN9780387276007.
- ^Brady, S.; Siegel, G.; Albers, R. W.; Price, D. (2005).Basic Neurochemistry: Molecular, Cellular and Medical Aspects.Academic Press. p. 348.ISBN9780080472072.
- ^abCarlomagno, G.; Unfer, V.; Roseff, S. (2011)."The D-chiro-inositol paradox in the ovary ".Fertility and Sterility.95(8): 2515–6.doi:10.1016/j.fertnstert.2011.05.027.PMID21641593.
- ^"Phytic acid".www.phytochemicals.info.Archivedfrom the original on 7 March 2018.Retrieved2018-05-02.
- ^Klopfenstein, T. J.; Angel, R.; Cromwell, G.; Erickson, G. E.; Fox, D. G.; Parsons, C.; Satter, L. D.; Sutton, A. L.; Baker, D. H. (July 2002)."Animal diet modification to decrease the potential for nitrogen and phosphorus pollution".Council for Agricultural Science and Technology.21.Archivedfrom the original on 2011-06-11.
- ^Hurrell, R. F. (September 2003)."Influence of vegetable protein sources on trace element and mineral bioavailability".The Journal of Nutrition.133(9): 2973S–2977S.doi:10.1093/jn/133.9.2973S.PMID12949395.
- ^Committee on Food Protection; Food and Nutrition Board; National Research Council (1973)."Phytates".Toxicants Occurring Naturally in Foods.National Academy of Sciences. pp.363–371.ISBN978-0-309-02117-3.
- ^abcClements, R. S.; Darnell, B. (September 1980)."myo-Inositol content of common foods: development of a high-myo-inositol diet ".The American Journal of Clinical Nutrition.33(9): 1954–1967.doi:10.1093/ajcn/33.9.1954.PMID7416064.S2CID4442333.
- ^"Phytic acid".www.phytochemicals.info.Archivedfrom the original on 2017-08-06.Retrieved2017-10-02.
- ^Larner, J. (2002)."D-chiro-Inositol—its functional role in insulin action and its deficit in insulin resistance ".International Journal of Experimental Diabetes Research.3(1): 47–60.doi:10.1080/15604280212528.PMC2478565.PMID11900279.
- ^Gerasimenko, J. V.; Flowerdew, S. E.; Voronina, S. G.; Sukhomlin, T. K.; Tepikin, A. V.; Petersen, O. H.; Gerasimenko, O. V. (December 2006)."Bile acids induce Ca2+release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors ".The Journal of Biological Chemistry.281(52): 40154–40163.doi:10.1074/jbc.M606402200.PMID17074764.
- ^Kukuljan, M.; Vergara, L.; Stojilković, S. S. (February 1997)."Modulation of the kinetics of inositol 1,4,5-trisphosphate-induced [Ca2+]i oscillations by calcium entry in pituitary gonadotrophs ".Biophysical Journal.72(2 Pt 1): 698–707.Bibcode:1997BpJ....72..698K.doi:10.1016/S0006-3495(97)78706-X.PMC1185595.PMID9017197.
- ^Rapiejko, P. J.; Northup, J. K.; Evans, T.; Brown, J. E.; Malbon, C. C. (November 1986)."G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate".The Biochemical Journal.240(1): 35–40.doi:10.1042/bj2400035.PMC1147372.PMID3103610.
- ^Shen, X.; Xiao, H.; Ranallo, R.; Wu, W.-H.; Wu, C. (January 2003)."Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates".Science.299(5603): 112–114.Bibcode:2003Sci...299..112S.doi:10.1126/science.1078068.PMID12434013.S2CID8381889.
- ^Steger, D. J.; Haswell, E. S.; Miller, A. L.; Wente, S. R.; O'Shea, E. K. (January 2003)."Regulation of chromatin remodeling by inositol polyphosphates".Science.299(5603): 114–116.Bibcode:2003Sci...299..114S.doi:10.1126/science.1078062.PMC1458531.PMID12434012.
- ^Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2000).Biochemistry(3rd ed.). San Francisco, CA: Benjamin Cummings. p. 855.ISBN978-0805330663.OCLC42290721.
- ^Laurence, W. L. (April 17, 1936)."Corn by-product yields explosive".The New York Times.p. 7. Archived fromthe originalon 2013-05-12.
- ^Ledgard, J. (2007).The Preparatory Manual of Explosives.Ledgard. p. 366.ISBN9780615142906.
- ^Chatterjee, J.; Majumder, A. L. (September 2010). "Salt-induced abnormalities on root tip mitotic cells ofAllium cepa:prevention by inositol pretreatment ".Protoplasma.245(1–4): 165–172.doi:10.1007/s00709-010-0170-4.PMID20559853.S2CID9128286.
- ^Sani, Gabriele; Gualtieri, Ida; Paolini, Marco; et al. (25 July 2019)."Drug Treatment of Trichotillomania (Hair-Pulling Disorder), Excoriation (Skin-picking) Disorder, and Nail-biting (Onychophagia)".Current Neuropharmacology.17(8): 775–786.doi:10.2174/1570159X17666190320164223.PMC7059154.PMID30892151.
- ^abTabrizi R, Ostadmohammadi V, Asemi Z (2018)."The effects of inositol supplementation on lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials".Lipids in Health and Disease.17(1): 123.doi:10.1186/s12944-018-0779-4.PMC5968598.PMID29793496.
- ^abcBenvenga S, Nordio M, Laganà AS, Unfer V (2021)."The Role of Inositol in Thyroid Physiology and in Subclinical Hypothyroidism Management".Frontiers in Endocrinology.12:662582.doi:10.3389/fendo.2021.662582.PMC8143049.PMID34040582.
- ^Howlett, Alexandra; Ohlsson, Arne; Plakkal, Nishad (8 July 2019)."Inositol in preterm infants at risk for or having respiratory distress syndrome".The Cochrane Database of Systematic Reviews.7(7): CD000366.doi:10.1002/14651858.CD000366.pub4.ISSN1469-493X.PMC6613728.PMID31283839.
- ^Cavalli, P.; Ronda, E. (2017)."Myoinositol: the bridge (PONTI) to reach a healthy pregnancy".International Journal of Endocrinology.2017:5846286.doi:10.1155/2017/5846286.PMC5274721.PMID28243254.
- ^Monastra, G.; Unfer, V.; Harrath, A. H.; Bizzarri, M. (January 2017). "Combining treatment withmyo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients ".Gynecological Endocrinology.33(1): 1–9.doi:10.1080/09513590.2016.1247797.hdl:11573/944617.PMID27898267.S2CID24836559.
- ^Nordio, M.; Proietti, E. (May 2012). "The combined therapy withmyo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared tomyo-inositol supplementation alone ".European Review for Medical and Pharmacological Sciences.16(5): 575–581.PMID22774396.
- ^Unfer, V.; et al. (2012). "Effects ofmyo-inositol in women with PCOS: a systematic review of randomized controlled trials ".Gynecological Endocrinology.28(7): 509–15.doi:10.3109/09513590.2011.650660.PMID22296306.S2CID24582338.
- ^Ciotta, L.; et al. (2011). "Effects ofmyo-inositol supplementation on oocyte's quality in PCOS patients: a double blind trial ".European Review for Medical and Pharmacological Sciences.15(5): 509–14.PMID21744744.
- ^Papaleo, E.; et al. (2009). "Contribution ofmyo-inositol to reproduction ".European Journal of Obstetrics & Gynecology and Reproductive Biology.147(2): 120–3.doi:10.1016/j.ejogrb.2009.09.008.PMID19800728.
- ^Isabella, R.; Raffone, E. (2012)."Does ovary need D-chiro-inositol? ".Journal of Ovarian Research.5(1): 14.doi:10.1186/1757-2215-5-14.PMC3447676.PMID22587479.(This paper currently has anexpression of concern,seedoi:10.1186/s13048-018-0431-y,PMID29976256,Retraction Watch)
- ^Moghetti, P. (2016). "Insulin resistance and polycystic ovary syndrome".Current Pharmaceutical Design.22(36): 5526–5534.doi:10.2174/1381612822666160720155855.PMID27510482.
- ^Facchinetti, F.; et al. (2015). "Results from the International Consensus Conference onmyo-Inositol and D-chiro-Inositol in Obstetrics and Gynecology: the link between metabolic syndrome and PCOS ".European Journal of Obstetrics & Gynecology and Reproductive Biology.195:72–6.doi:10.1016/j.ejogrb.2015.09.024.PMID26479434.
- ^Colazingari, S.; et al. (2013). "The combined therapymyo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial ".Archives of Gynecology and Obstetrics.288(6): 1405–11.doi:10.1007/s00404-013-2855-3.PMID23708322.S2CID45611717.
- ^Kamenov, Z.; et al. (2015). "Ovulation induction withmyo-inositol alone and in combination with clomiphene citrate in polycystic ovarian syndrome patients with insulin resistance ".Gynecological Endocrinology.31(2): 131–5.doi:10.3109/09513590.2014.964640.PMID25259724.S2CID24469378.
- ^González, F. (2012)."Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction".Steroids.77(4): 300–5.doi:10.1016/j.steroids.2011.12.003.PMC3309040.PMID22178787.
- ^Monastra, G.; et al. (2018). "alpha-Lactalbumin effect onmyo-inositol intestinal absorption: in vivo and in vitro ".Current Drug Delivery.15(9): 1305–1311.doi:10.2174/1567201815666180509102641.PMID29745333.S2CID13691602.
- ^Oliva, M. M.; et al. (2018)."Effects ofmyo-inositol plusalpha-lactalbumin inmyo-inositol-resistant PCOS women ".Journal of Ovarian Research.11(1): 38.doi:10.1186/s13048-018-0411-2.PMC5944130.PMID29747700.
- ^"Inositol, Nerve guidance, Cutting agent manufacturer".Tianyu Feed Additive.Archivedfrom the original on 2014-09-08.Retrieved2013-07-21.
- ^Golianopoulos, T. (2012-05-12)."Drug doubles: What actors actually toke, smoke and snort on camera".Wired.Archivedfrom the original on 2012-05-14.Retrieved2012-05-14.
- ^How Fake Drugs Are Made For Movies | Movies Insider,retrieved2022-09-26
- ^abAwuchi, Chinaza (2017)."Sugar Alcohols: Chemistry, Production, Health Concerns and Nutritional Importance of Mannitol, Sorbitol, Xylitol, and Erythritol".International Journal of Advanced Academic Research.5(11): 1954–1967.
- ^Borgi MA, Boudebbouze S, Mkaouar H, Maguin E, Rhimi M (2015)."Bacillus phytases: Current status and future prospects".Bioengineered.5(4): 233–236.doi:10.1080/21655979.2015.1048050.PMC4601277.PMID25946551.
- ^Stentz R, Osborne S, Horn N, Li AW, Hautefort I, Bongaerts R, Rouyer M, Bailey P, Shears SB, Hemmings AM, Brearley CA, Carding SR (27 February 2014)."A Bacterial Homolog of a Eukaryotic Inositol Phosphate Signaling Enzyme Mediates Cross-kingdom Dialog in the Mammalian Gut".Cell Reports.6(4): 646–656.doi:10.1016/j.celrep.2014.01.021.PMC3969271.PMID24529702.
- ^Ehlers, Anke; Marakis, Georgios; Lampen, Alfonso; Hirsch-Ernst, Karen Ildico (2019-08-01)."Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe".Food and Chemical Toxicology.130:109–121.doi:10.1016/j.fct.2019.05.028.ISSN0278-6915.PMID31112702.
- ^DiSalvo, David."We Know About Caffeine in Energy Drinks Like Monster, But What About the Other Ingredients?".Forbes.Retrieved2020-12-22.

