Myristoylation

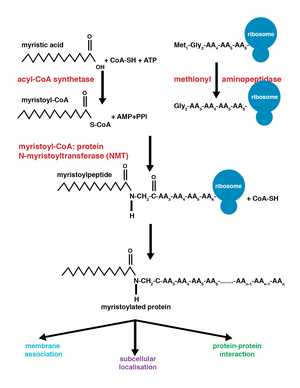
Myristoylationis a lipidation modification where amyristoyl group,derived frommyristic acid,iscovalentlyattached by anamide bondto the alpha-amino group of anN-terminalglycineresidue.[1]Myristic acid is a 14-carbon saturated fatty acid (14:0) with the systematic name ofn-tetradecanoic acid. This modification can be added either co-translationally orpost-translationally.N-myristoyltransferase (NMT)catalyzes themyristic acidaddition reaction in thecytoplasmof cells.[2]This lipidation event is the most common type of fatty acylation[3]and is present in many organisms, includinganimals,plants,fungi,protozoans[4]andviruses.Myristoylation allows for weak protein–protein and protein–lipid interactions[5]and plays an essential role in membrane targeting,protein–protein interactionsand functions widely in a variety ofsignal transductionpathways.
Discovery
[edit]In 1982, Koiti Titani's lab identified an "N-terminal blocking group "on the catalytic subunit ofcyclic AMP-dependent protein kinasein cows asn-tetradecanoyl.[6]Almost simultaneously in Claude B. Klee's lab, this sameN-terminal blocking group was further characterized as myristic acid.[7]Both labs made this discovery utilizing similar techniques:mass spectrometryandgas chromatography.[6][7]
N-myristoyltransferase
[edit]
The enzymeN-myristoyltransferase (NMT) orglycylpeptideN-tetradecanoyltransferaseis responsible for the irreversible addition of a myristoyl group toN-terminal or internal glycine residues of proteins. This modification can occur co-translationally orpost-translationally.In vertebrates, this modification is carried about by two NMTs,NMT1andNMT2,both of which are members of the GCN5acetyltransferasesuperfamily.[8]
Structure
[edit]Thecrystal structureof NMT reveals two identical subunits, each with its own myristoyl CoA binding site. Each subunit consists of a large saddle-shapedβ-sheetsurrounded byα-helices.The symmetry of the fold is pseudo twofold.[clarification needed]Myristoyl CoA binds at theN-terminalportion, while theC-terminalend binds the protein.[9]
Mechanism
[edit]The addition of the myristoyl group proceeds via anucleophilic addition-elimination reaction.First, myristoylcoenzyme A (CoA)is positioned in its binding pocket of NMT so that thecarbonylfaces two amino acid residues,phenylalanine170 andleucine171.[9]This polarizes the carbonyl so that there is a net positive charge on the carbon, making it susceptible to nucleophilic attack by theglycineresidue of the protein to be modified. When myristoyl CoA binds, NMT reorients to allow binding of the peptide. TheC-terminus of NMT then acts as a general base todeprotonatethe NH3+,activating theamino groupto attack at thecarbonyl groupof myristoyl-CoA. The resultingtetrahedral intermediateis stabilized by the interaction between a positively chargedoxyanion holeand the negatively chargedalkoxideanion. Free CoA is then released, causing aconformational changein the enzyme that allows the release of the myristoylated peptide.[2]

Co-translational vs. post-translational addition
[edit]Co-translational and post-translational covalent modifications enable proteins to develop higher levels of complexity in cellular function, further adding diversity to theproteome.[10]The addition of myristoyl-CoA to a protein can occur during protein translation or after. During co-translational addition of the myristoyl group, theN-terminalglycineis modified following cleavage of theN-terminalmethionineresidue in the newly forming, growingpolypeptide.[1]Post-translational myristoylation typically occurs following acaspasecleavage event, resulting in the exposure of an internal glycine residue, which is then available for myristic acid addition.[8]
Functions
[edit]Myristoylated proteins
[edit]| Protein | Physiological Role | Myristoylation Function |
|---|---|---|
| Actin | Cytoskeletonstructural protein | Post-translational myristoylation during apoptosis[8] |
| Bid | Apoptosis promoting protein | Post-translational myristoylation after caspase cleavage targets protein tomitochondrial membrane[8] |
| MARCKS | actin cross-linking when phosphorylated by protein kinase C | Co-translational myristoylation aids in plasma membrane association |
| G-Protein | SignalingGTPase | Co-translational myristoylation aids in plasma membrane association[11] |
| Gelsolin | Actin filament-severing protein | Post-translational myristoylation up-regulates anti-apoptotic properties[8] |
| PAK2 | Serine/threonine kinasecell growth, mobility, survival stimulator | Post-translational myristoylation up-regulates apoptotic properties and inducesplasma membranelocalization[8] |
| Arf | vesiculartrafficking and actin remodeling regulation | N-terminus myristoylation aids in membrane association |
| Hippocalcin | Neuronal calcium sensor | Contains a Ca2+/myristoyl switch |
| FSP1 | Apoptosis-inducing factor mitochondria-associated 2 (AIFM2) | Facilitates the association of FSP1 with the lipid-bilayer which enables ferroptosis resistance.[12] |
Myristoylation molecular switch
[edit]Myristoylation not only diversifies the function of a protein, but also adds layers of regulation to it. One of the most common functions of the myristoyl group is inmembrane associationandcellular localizationof the modified protein. Though the myristoyl group is added onto the end of the protein, in some cases it is sequestered withinhydrophobicregions of the protein rather than solvent exposed.[5]By regulating the orientation of the myristoyl group, these processes can be highly coordinated and closely controlled. Myristoylation is thus a form of "molecular switch."[13]
Both hydrophobic myristoyl groups and "basic patches" (highly positive regions on the protein) characterize myristoyl-electrostatic switches. The basic patch allows for favorableelectrostatic interactionsto occur between the negatively chargedphospholipid headsof the membrane and the positive surface of the associating protein. This allows tighter association and directed localization of proteins.[5]
Myristoyl-conformational switches can come in several forms.Ligand bindingto a myristoylated protein with its myristoyl group sequestered can cause aconformational changein the protein, resulting in exposure of the myristoyl group. Similarly, some myristoylated proteins are activated not by a designated ligand, but by the exchange ofGDPforGTPbyguanine nucleotide exchange factorsin the cell. Once GTP is bound to the myristoylated protein, it becomes activated, exposing the myristoyl group. These conformational switches can be utilized as a signal for cellular localization, membrane-protein, andprotein–protein interactions.[5][13][14]
Dual modifications of myristoylated proteins
[edit]Further modifications onN-myristoylated proteins can add another level of regulation for myristoylated protein. Dualacylationcan facilitate more tightly regulated protein localization, specifically targeting proteins tolipid raftsat membranes[15]or allowing dissociation of myristoylated proteins from membranes.
Myristoylation andpalmitoylationare commonly coupled modifications. Myristoylation alone can promote transient membrane interactions[5]that enable proteins to anchor to membranes but dissociate easily. Further palmitoylation allows for tighter anchoring and slower dissociation from membranes when required by the cell. This specific dual modification is important forG protein-coupled receptorpathways and is referred to as the dual fatty acylation switch.[5][8]
Myristoylation is often followed byphosphorylationof nearby residues. Additional phosphorylation of the same protein can decrease the electrostatic affinity of the myristoylated protein for the membrane, causingtranslocationof that protein to the cytoplasm following dissociation from the membrane.[5]
Signal transduction
[edit]Myristoylation plays a vital role in membrane targeting andsignal transduction[16]in plant responses to environmental stress. In addition, in signal transduction via G protein,palmitoylationof the α subunit,prenylationof the γ subunit, and myristoylation is involved in tethering the G protein to the inner surface of the plasma membrane so that the G protein can interact with its receptor.[17]
Apoptosis
[edit]Myristoylation is an integral part ofapoptosis,or programmed cell death. Apoptosis is necessary for cell homeostasis and occurs when cells are under stress such ashypoxiaorDNA damage.Apoptosis can proceed by either mitochondrial or receptor mediated activation. In receptor mediated apoptosis, apoptotic pathways are triggered when the cell binds a death receptor. In one such case, death receptor binding initiates the formation of thedeath-inducing signaling complex,a complex composed of numerous proteins including several caspases, includingcaspase 3.Caspase 3 cleaves a number of proteins that are subsequently myristoylated by NMT. Thepro-apoptotic BH3-interacting domain death agonist (Bid)is one such protein that once myristoylated, translocates to themitochondriawhere it prompts the release ofcytochrome cleading to cell death.[8]Actin,gelsolinand p21-activated kinase 2PAK2are three other proteins that are myristoylated following cleavage bycaspase 3,which leads to either the up-regulation or down-regulation of apoptosis.[8]
Impact on human health
[edit]Cancer
[edit]c-Srcis a gene that codes for proto-oncogene tyrosine-protein kinase Src, a protein important for normalmitotic cycling.It is phosphorylated and dephosphorylated to turn signaling on and off. Proto-oncogene tyrosine-protein kinase Src must be localized to theplasma membranein order to phosphorylate other downstream targets; myristoylation is responsible for thismembrane targetingevent. Increased myristoylation ofc-Srccan lead to enhancedcell proliferationand be responsible fortransforming normal cells into cancer cells.[5][14][18]Activation ofc-Srccan lead to the so-called "hallmarks of cancer",among them upregulation ofangiogenesis,proliferation, andinvasion.[19]
Viral infectivity
[edit]
HIV-1is aretrovirusthat relies on myristoylation of one of its structural proteins in order to successfully package its genome, assemble and mature into a new infectious particle.Viral matrix protein,theN-terminal–most domain of thegag polyprotein,is myristoylated.[20]This myristoylation modification targets gag to the membrane of the host cell. Utilizing the myristoyl-electrostatic switch,[13]including a basic patch on the matrix protein,gagcan assemble atlipid raftsat theplasma membraneforviralassembly, budding and further maturation.[18]In order to prevent viral infectivity, myristoylation of the matrix protein could become a good drug target.
Prokaryotic and eukaryotic infections
[edit]Certain NMTs are therapeutic targets for development of drugs against bacterialinfections.Myristoylation has been shown to be necessary for the survival of a number of disease-causingfungi,among themC. albicansandC. neoformans.In addition toprokaryoticbacteria, the NMTs of numerous disease-causingeukaryoticorganisms have been identified asdrug targetsas well. Proper NMT functioning in theprotozoaLeishmania majorandLeishmania donovani(leishmaniasis),Trypanosoma brucei(African sleeping sickness), andP. falciparum(malaria) is necessary for survival of the parasites. Inhibitors of these organisms are under current investigation. Apyrazolesulfonamideinhibitorhas been identified that selectively bindsT. brucei,competing for thepeptide bindingsite, thus inhibiting enzymatic activity and eliminating the parasite from the bloodstream of mice withAfrican sleeping sickness.[8]
See also
[edit]References
[edit]- ^abCox, David L. Nelson, Michael M. (2005).Lehninger principles of biochemistry(4th ed.). New York: W.H. Freeman.ISBN978-0716743392.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^abTamanoi, Fuyuhiko; Sigman, David S., eds. (2001).Protein lipidation.Vol. 21 (3rd ed.). San Diego, CA: Academic Press.ISBN978-0-12-122722-7.
- ^Mohammadzadeh, Fatemeh; Hosseini, Vahid; Mehdizadeh, Amir; Dani, Christian; Darabi, Masoud (2018-11-30)."A method for the gross analysis of global protein acylation by gas-liquid chromatography".IUBMB Life.71(3): 340–346.doi:10.1002/iub.1975.ISSN1521-6543.PMID30501005.
- ^Kara, UA; Stenzel, DJ; Ingram, LT; Bushell, GR; Lopez, JA; Kidson, C (Apr 1988)."Inhibitory monoclonal antibody against a (myristylated) small-molecular-weight antigen from Plasmodium falciparum associated with the parasitophorous vacuole membrane".Infection and Immunity.56(4): 903–9.doi:10.1128/IAI.56.4.903-909.1988.PMC259388.PMID3278984.
- ^abcdefghFarazi, T. A. (29 August 2001)."The Biology and Enzymology of ProteinN-Myristoylation ".Journal of Biological Chemistry.276(43): 39501–39504.doi:10.1074/jbc.R100042200.PMID11527981.
- ^abCarr, SA; Biemann, K; Shoji, S; Parmelee, DC; Titani, K (Oct 1982)."N-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle ".Proceedings of the National Academy of Sciences of the United States of America.79(20): 6128–31.Bibcode:1982PNAS...79.6128C.doi:10.1073/pnas.79.20.6128.PMC347072.PMID6959104.
- ^abAitken, A; Cohen, P; Santikarn, S; Williams, DH; Calder, AG; Smith, A; Klee, CB (Dec 27, 1982)."Identification of the NH2-terminal blocking group of calcineurin B as myristic acid ".FEBS Letters.150(2): 314–8.doi:10.1016/0014-5793(82)80759-x.PMID7160476.S2CID40889752.
- ^abcdefghijMartin, Dale D.O.; Beauchamp, Erwan; Berthiaume, Luc G. (January 2011). "Post-translational myristoylation: Fat matters in cellular life and death".Biochimie.93(1): 18–31.doi:10.1016/j.biochi.2010.10.018.PMID21056615.
- ^abBhatnagar, RS; Fütterer, K; Waksman, G; Gordon, JI (Nov 23, 1999). "The structure of myristoyl-CoA:proteinN-myristoyltransferase ".Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.1441(2–3): 162–72.doi:10.1016/s1388-1981(99)00155-9.PMID10570244.
- ^Snider, Jared."Overview of Post-Translational Modifications (PTMs)".Thermo Scientific.
- ^Chen, Katherine A.; Manning, David R. (2001)."Regulation of G proteins by covalent modification".Oncogene.20(13): 1643–1652.doi:10.1038/sj.onc.1204185.PMID11313912.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Doll, Sebastian; Freitas, Florencio Porto; Shah, Ron; Aldrovandi, Maceler; da Silva, Milene Costa; Ingold, Irina; Goya Grocin, Andrea; Xavier da Silva, Thamara Nishida; Panzilius, Elena; Scheel, Christina H.; Mourão, André (November 2019)."FSP1 is a glutathione-independent ferroptosis suppressor".Nature.575(7784): 693–698.Bibcode:2019Natur.575..693D.doi:10.1038/s41586-019-1707-0.hdl:10044/1/75345.ISSN1476-4687.PMID31634899.S2CID204833583.
- ^abcMcLaughlin, Stuart; Aderem, Alan (July 1995). "The myristoyl-electrostatic switch: a modulator of reversible protein–membrane interactions".Trends in Biochemical Sciences.20(7): 272–276.doi:10.1016/S0968-0004(00)89042-8.PMID7667880.
- ^abWright, Megan H.; Heal, William P.; Mann, David J.; Tate, Edward W. (7 November 2009)."Protein myristoylation in health and disease".Journal of Chemical Biology.3(1): 19–35.doi:10.1007/s12154-009-0032-8.PMC2816741.PMID19898886.
- ^Levental, Ilya; Grzybek, Michal; Simons, Kai (3 August 2010). "Greasing Their Way: Lipid Modifications Determine Protein Association with Membrane Rafts".Biochemistry.49(30): 6305–6316.doi:10.1021/bi100882y.PMID20583817.
- ^HAYASHI, Nobuhiro; TITANI, Koiti (2010)."N-myristoylated proteins, key components in intracellular signal transduction systems enabling rapid and flexible cell responses ".Proceedings of the Japan Academy, Series B.86(5): 494–508.Bibcode:2010PJAB...86..494H.doi:10.2183/pjab.86.494.PMC3108300.PMID20467215.
- ^Wall, Mark A.; Coleman, David E.; Lee, Ethan; Iñiguez-Lluhi, Jorge A.; Posner, Bruce A.; Gilman, Alfred G.; Sprang, Stephen R. (December 1995)."The structure of the G protein heterotrimer Giα1β1γ2".Cell.83(6): 1047–1058.doi:10.1016/0092-8674(95)90220-1.PMID8521505.
- ^abShoji, S; Kubota, Y (Feb 1989)."[Function of protein myristoylation in cellular regulation and viral proliferation]".Yakugaku Zasshi.109(2): 71–85.doi:10.1248/yakushi1947.109.2_71.PMID2545855.
- ^Hanahan, Douglas; Weinberg, Robert A. (March 2011)."Hallmarks of Cancer: The Next Generation".Cell.144(5): 646–674.doi:10.1016/j.cell.2011.02.013.PMID21376230.
- ^Hearps, AC; Jans, DA (Mar 2007). "Regulating the functions of the HIV-1 matrix protein".AIDS Research and Human Retroviruses.23(3): 341–6.doi:10.1089/aid.2006.0108.PMID17411366.


