NADPH oxidase
| NAD(P)H oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.6.3.1 | ||||||||
| CAS no. | 77106-92-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDBstructures | RCSB PDBPDBePDBsum | ||||||||
| |||||||||
| Ferric reductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | NADPH oxidase | ||||||||
| Pfam | PF01794 | ||||||||
| InterPro | IPR013130 | ||||||||
| TCDB | 5.B.1 | ||||||||
| OPM superfamily | 464 | ||||||||
| OPM protein | 5o05 | ||||||||
| |||||||||
NADPHoxidase(nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-boundenzymecomplex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes ofphagosomesused byneutrophilwhite blood cells to engulf microorganisms. Humanisoformsof the catalytic component of the complex includeNOX1,NOX2,NOX3,NOX4,NOX5,DUOX1,andDUOX2.[1]
Reaction[edit]
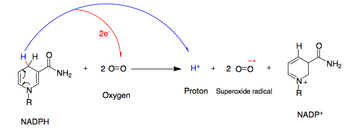
NADPH oxidase catalyzes the production of asuperoxidefree radical by transferring one electron tooxygenfromNADPH.[2]
- NADPH + 2O2↔ NADP++ 2O−2+ H+
Types[edit]
In mammals, NADPH oxidase is found in two types: one inwhite blood cells(neutrophilic) and the other invascularcells, differing in biochemical structure and functions.[3]Neutrophilic NADPH oxidase produces superoxide almost instantaneously, whereas the vascular enzyme produces superoxide in minutes to hours.[4]Moreover, in white blood cells, superoxide has been found to transfer electrons across the membrane to extracellular oxygen, while in vascular cells, the radical anion appears to be released mainly intracellularly.[5][6]
Neutrophilic type[edit]
The isoform found in neutrophils is made up of six subunits. These subunits are:
- aRho GTPase,usuallyRac1orRac2(Rac stands for Rho-related C3botulinum toxinsubstrate)
- Fivephagocyticoxidase subunits:
Vascular type[edit]
There are several vascular isoforms of the complex which use paralogs the NOX2 subunit:
Thyroid type[edit]
There are two further paralogs of NOX2 subunit in the thyroid:
Structure[edit]
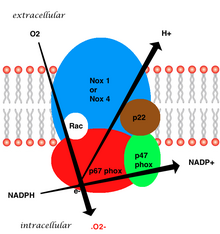
The whole structure of the membrane-bound vascular enzyme is composed of five parts: twocytosolicsubunits (p47phox and p67phox), a cytochrome b558 which consists of gp91phox, p22phox and a small G protein Rac.[3]Generation of the superoxide in vascular NADPH occurs by a one-electron reduction of oxygen via the gp91phox subunit, using reduced NADPH as the electron donor. The small G protein carries an essential role in the activation of the oxidase by switching between a GDP-bound (inactive) and GTP-linked (active) forms.[8]
Biological function[edit]
NADPH oxidases (NOXes) are one of the major sources of cellularreactive oxygen species (ROS),and they still are the focus of extensive research interest due to their exclusive function in producing ROS under normal physiological conditions. The NADPH oxidase complex is dormant under normal circumstances but is activated to assemble in the membranes duringrespiratory burst.The activated NADPH oxidase generates superoxide which has roles in animal immune response and plant signalling.[9]
Superoxide can be produced inphagosomeswhich have ingestedbacteriaandfungi,or it can be produced outside of the cell.[10]In macrophages, superoxide killsbacteriaand fungi by mechanisms that are not yet fully understood.[11][12]Superoxide spontaneously dismutates to form peroxide which is then protonated to produce hydrogen peroxide. Opinions are polarised as to how the oxidase kills microbes in neutrophils. On the one hand it is thought that hydrogen peroxide acts as substrate for myeloperoxidase to produce hypochlorous acid.[13]It may also inactivate critical metabolic enzymes, initiatelipid peroxidation,damageiron-sulphur clusters,[14]and liberate redox-active iron, which allows the generation of indiscriminate oxidants such as the hydroxyl radical.[12]An alternative view is that the oxidase elevates the pH in the vacuole to about 9.0, which is optimal for the neutral proteases that degranulate from the cytoplasmic granules (where they are inactive at pH ~5.5) and it pumps potassium into the vacuole, which solubilises the enzymes, and it is the activated proteases that kill and digest the microbes.[15]
Ininsects,NOXes had some functions clarified.Arthropodshave three NOX types (NOX4-art, an arthropod-specific p22-phox-independent NOX4, and two calcium-dependent enzymes, DUOX).[16][17][18]In the gut, DUOX-dependent ROS production from bacteria-stimulatedDrosophila melanogastermucosa is an important pathogen-killing mechanism[19]and can increase defecation as a defense response.[20]InAedes aegypti,DUOX is involved in the control of the gut indigenous microbiota.[21]Rhodnius prolixushas calcium activated DUOX, which is involved in eggshell hardening,[22]and NOX5, which is involved in the control of gut motility and blood digestion.[23][24]
Regulation[edit]
Careful regulation of NADPH oxidase activity is crucial to maintain a healthy level of ROS in the body. The enzyme is dormant in resting cells but becomes rapidly activated by several stimuli, including bacterial products and cytokines.[25]Vascular NADPH oxidases are regulated by a variety of hormones and factors known to be important players in vascular remodeling and disease. These includethrombin,platelet-derived growth factor(PDGF),tumor necrosis factor(TNFa),lactosylceramide,interleukin-1,and oxidizedLDL.[26]It is also stimulated by agonists andarachidonic acid.[26]Conversely, assembly of the complex can be inhibited byapocyninanddiphenylene iodonium.Apocynin decreases influenza-induced lung inflammation in micein vivoand so may have clinical benefits in the treatment of influenza.[27]
Ang-1 triggers NOX2, NOX4, and the mitochondria to release ROS and that ROS derived from these sources play distinct roles in the regulation of the Ang-1/Tie 2 signaling pathway and pro-angiogenic responses.[28]
Pathology[edit]
Superoxides are crucial in killing foreign bacteria in the human body. Consequently, under-activity can lead to an increased susceptibility to organisms such as catalase-positive microbes, and over-activity can lead tooxidative stressand cell damage.
Excessive production of ROS in vascular cells causes many forms of cardiovascular disease includinghypertension,atherosclerosis,myocardial infarction,andischemic stroke.[29]Atherosclerosis is caused by the accumulation of macrophages containing cholesterol (foam cells) in artery walls (in theintima). ROS produced by NADPH oxidase activate an enzyme that makes the macrophages adhere to the artery wall (by polymerizing actin fibers). This process is counterbalanced by NADPH oxidase inhibitors, and by antioxidants. An imbalance in favor of ROS produces atherosclerosis. In vitro studies have found that the NADPH oxidase inhibitors apocynin and diphenyleneiodonium, along with the antioxidants N-acetyl-cysteine and resveratrol, depolymerized the actin, broke the adhesions, and allowed foam cells to migrate out of the intima.[30][31]
One study suggests a role for NADPH oxidase inketamine-induced loss of neuronalparvalbuminandGAD67expression.[32]Similar loss is observed inschizophrenia,and the results may point at the NADPH oxidase as a possible player in the pathophysiology of the disease.[33]Nitro blue tetrazoliumis used in a diagnostic test, in particular, for chronic granulomatous disease, a disease in which there is a defect in NADPH oxidase; therefore, the phagocyte is unable to make the reactive oxygen species or radicals required for bacterial killing, resulting in bacteria thriving within the phagocyte. The higher the blue score the better the cell is at producing reactive oxygen species.
It has also been shown that NADPH oxidase plays a role in the mechanism that induces the formation ofsFlt-1,a protein that deactivates certain proangiogenic factors that play a role in the development of the placenta, by facilitating the formation ofreactive oxygen species,which are suspected intermediaries in sFlt-1 formation. These effects are in part responsible for inducing pre-eclampsia in pregnant women[34]
Mutations[edit]
Mutations in the NADPH oxidase subunit genes cause severalChronic Granulomatous Diseases(CGD), characterized by extreme susceptibility to infection.[26]These include:
- X-linked chronic granulomatous disease(CGD)
- Autosomal recessive cytochrome b-negative CGD
- Autosomal recessive cytochrome b-positive CGD type I
- Autosomal recessive cytochrome b-positive CGD type II.
In these diseases, cells have a low capacity for phagocytosis, and persistent bacterial infections occur. Areas of infected cells are common, granulomas. A similar disorder calledneutrophil immunodeficiency syndromeis linked to a mutation in the RAC2, also a part of the complex.
Inhibition[edit]
NADPH oxidase can be inhibited byapocynin,nitric oxide(NO), anddiphenylene iodonium.Apocynin acts by preventing the assembly of the NADPH oxidase subunits. Apocynin decreases influenza-induced lung inflammation in micein vivoand so may have clinical benefits in the treatment of influenza.[27]
Inhibition of NADPH oxidase by NO blocks the source of oxidative stress in the vasculature. NO donor drugs (nitrovasodilators) have therefore been used for more than a century to treatcoronary artery disease,hypertension,andheart failureby preventing excess superoxide from deteriorating healthy vascular cells.[3]
More advanced NADPH oxidase inhibitors includeGKT-831(FormerlyGKT137831), a dual Inhibitor of isoforms NOX4 and NOX1[35]which was patented in 2007.[36]The compound was initially developed forIdiopathic pulmonary fibrosisand obtainedorphan drugdesignation by theFDAandEMAat end of 2010.[37]
References[edit]
- ^Sahoo S, Meijles DN, Pagano PJ (March 2016)."NADPH oxidases: key modulators in aging and age-related cardiovascular diseases?".Clinical Science.130(5): 317–335.doi:10.1042/CS20150087.PMC4818578.PMID26814203.
- ^Panday A, Sahoo MK, Osorio D, Batra S (January 2015)."NADPH oxidases: an overview from structure to innate immunity-associated pathologies".Cellular & Molecular Immunology.12(1): 5–23.doi:10.1038/cmi.2014.89.PMC4654378.PMID25263488.
- ^abcDusting GJ, Selemidis S, Jiang F (March 2005)."Mechanisms for suppressing NADPH oxidase in the vascular wall".Memórias do Instituto Oswaldo Cruz.100(Suppl 1): 97–103.doi:10.1590/S0074-02762005000900016.hdl:1807/8204.PMID15962105.
- ^Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK (August 1998)."Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts".Hypertension.32(2): 331–337.doi:10.1161/01.hyp.32.2.331.PMID9719063.
- ^Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW (June 1994)."Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells".Circulation Research.74(6): 1141–1148.doi:10.1161/01.res.74.6.1141.PMID8187280.
- ^Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. (September 1998)."Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy".Hypertension.32(3): 488–495.doi:10.1161/01.hyp.32.3.488.PMID9740615.
- ^Herb M (February 2024)."NADPH oxidase 3: Beyond the innear ear".Antioxidants.13(2).doi:10.3390/antiox13020219.PMC10886416.
- ^Heyworth PG, Knaus UG, Settleman J, Curnutte JT, Bokoch GM (November 1993)."Regulation of NADPH oxidase activity by Rac GTPase activating protein(s)".Molecular Biology of the Cell.4(11): 1217–1223.doi:10.1091/mbc.4.11.1217.PMC275755.PMID8305740.
- ^Sharma, Pallavi; Jha, Ambuj Bhushan; Dubey, Rama Shanker; Pessarakli, Mohammad (2012-04-24)."Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions".Journal of Botany.2012:1–26.doi:10.1155/2012/217037.ISSN2090-0120.
- ^Herb M, Gluschko A, Wiegmann K, Farid A, Wolf A, Utermöhlen O, et al. (February 2019)."Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO".Science Signaling.12(568): eaar5926.doi:10.1126/scisignal.aar5926.PMID30755476.
- ^Herb M, Schramm M (February 2021)."Functions of ROS in Macrophages and Antimicrobial Immunity".Antioxidants.10(2): 313.doi:10.3390/antiox10020313.PMC7923022.PMID33669824.
- ^abSlauch JM (May 2011)."How does the oxidative burst of macrophages kill bacteria? Still an open question".Molecular Microbiology.80(3): 580–583.doi:10.1111/j.1365-2958.2011.07612.x.PMC3109634.PMID21375590.
- ^Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM (February 2013)."Myeloperoxidase: a front-line defender against phagocytosed microorganisms".Journal of Leukocyte Biology.93(2): 185–198.doi:10.1189/jlb.0712349.PMC3545676.PMID23066164.
- ^Djaman O, Outten FW, Imlay JA (October 2004)."Repair of oxidized iron-sulfur clusters in Escherichia coli".The Journal of Biological Chemistry.279(43): 44590–44599.doi:10.1074/jbc.M406487200.PMID15308657.
- ^Levine AP, Segal AW (August 2016)."The NADPH Oxidase and Microbial Killing by Neutrophils, With a Particular Emphasis on the Proposed Antimicrobial Role of Myeloperoxidase within the Phagocytic Vacuole".Microbiology Spectrum.4(4).doi:10.1128/microbiolspec.MCHD-0018-2015.PMID27726789.
- ^Gandara AC, Torres A, Bahia AC, Oliveira PL, Schama R (March 2017)."Evolutionary origin and function of NOX4-art, an arthropod specific NADPH oxidase".BMC Evolutionary Biology.17(1): 92.Bibcode:2017BMCEE..17...92G.doi:10.1186/s12862-017-0940-0.PMC5372347.PMID28356077.
- ^Kawahara T, Quinn MT, Lambeth JD (July 2007)."Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes".BMC Evolutionary Biology.7(1): 109.Bibcode:2007BMCEE...7..109K.doi:10.1186/1471-2148-7-109.PMC1940245.PMID17612411.
- ^Gandara, Ana Caroline P.; Oliveira, Pedro L. (2023), Pick, Edgar (ed.),"NADPH Oxidases in Arthropods",NADPH Oxidases Revisited: From Function to Structure,Cham: Springer International Publishing, pp. 477–488,doi:10.1007/978-3-031-23752-2_28,ISBN978-3-031-23751-5,retrieved2023-10-19
- ^Ha EM, Oh CT, Bae YS, Lee WJ (November 2005). "A direct role for dual oxidase in Drosophila gut immunity".Science.310(5749): 847–850.Bibcode:2005Sci...310..847H.doi:10.1126/science.1117311.PMID16272120.S2CID12476863.
- ^Du EJ, Ahn TJ, Kwon I, Lee JH, Park JH, Park SH, et al. (January 2016). Miguel-Aliaga I (ed.)."TrpA1 Regulates Defecation of Food-Borne Pathogens under the Control of the Duox Pathway".PLOS Genetics.12(1): e1005773.doi:10.1371/journal.pgen.1005773.PMC4699737.PMID26726767.
- ^Oliveira JH, Gonçalves RL, Lara FA, Dias FA, Gandara AC, Menna-Barreto RF, et al. (March 2011). Schneider DS (ed.)."Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota".PLOS Pathogens.7(3): e1001320.doi:10.1371/journal.ppat.1001320.PMC3060171.PMID21445237.
- ^Dias FA, Gandara AC, Queiroz-Barros FG, Oliveira RL, Sorgine MH, Braz GR, Oliveira PL (December 2013)."Ovarian dual oxidase (Duox) activity is essential for insect eggshell hardening and waterproofing".The Journal of Biological Chemistry.288(49): 35058–35067.doi:10.1074/jbc.M113.522201.PMC3853258.PMID24174530.
- ^Montezano AC, De Lucca Camargo L, Persson P, Rios FJ, Harvey AP, Anagnostopoulou A, et al. (June 2018)."NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function".Journal of the American Heart Association.7(12).doi:10.1161/JAHA.118.009388.PMC6220544.PMID29907654.
- ^Gandara AC, Dias FA, de Lemos PC, Stiebler R, Bombaça AC, Menna-Barreto R, Oliveira PL (2021-02-25).""Urate and NOX5 Control Blood Digestion in the Hematophagous InsectRhodnius prolixus"".Frontiers in Physiology.12:633093.doi:10.3389/fphys.2021.633093.PMC7947236.PMID33716782.
- ^Geiszt M (July 2006)."NADPH oxidases: new kids on the block".Cardiovascular Research.71(2): 289–299.doi:10.1016/j.cardiores.2006.05.004.PMID16765921.
- ^abcGriendling KK, Sorescu D, Ushio-Fukai M (March 2000)."NAD(P)H oxidase: role in cardiovascular biology and disease".Circulation Research.86(5): 494–501.doi:10.1161/01.res.86.5.494.PMID10720409.
- ^abVlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, Selemidis S (February 2011)."Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation".PLOS Pathogens.7(2): e1001271.doi:10.1371/journal.ppat.1001271.PMC3033375.PMID21304882.
- ^Harel S, Mayaki D, Sanchez V, Hussain SN (May 2017). "NOX2, NOX4, and mitochondrial-derived reactive oxygen species contribute to angiopoietin-1 signaling and angiogenic responses in endothelial cells".Vascular Pharmacology.92:22–32.doi:10.1016/j.vph.2017.03.002.PMID28351775.
- ^Wattanapitayakul SK, Bauer JA (February 2001). "Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications".Pharmacology & Therapeutics.89(2): 187–206.doi:10.1016/S0163-7258(00)00114-5.PMID11316520.
- ^Park YM, Febbraio M, Silverstein RL (January 2009)."CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima".The Journal of Clinical Investigation.119(1): 136–145.doi:10.1172/JCI35535.PMC2613464.PMID19065049.
- ^Curtiss LK (March 2009). "Reversing atherosclerosis?".The New England Journal of Medicine.360(11): 1144–1146.doi:10.1056/NEJMcibr0810383.PMID19279347.
- ^Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL (December 2007). "Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase".Science.318(5856): 1645–1647.Bibcode:2007Sci...318.1645B.doi:10.1126/science.1148045.PMID18063801.S2CID41932041.
- ^Tom Fagan.Does Oxidative Stress Link NMDA and GABA Hypotheses of Schizophrenia?Archived2007-12-30 at theWayback MachineSchizophrenia Research Forum. December 09, 2007.
- ^Huang QT, Zhang M, Zhong M, Yu YH, Liang WZ, Hang LL, et al. (December 2013). "Advanced glycation end products as an upstream molecule triggers ROS-induced sFlt-1 production in extravillous trophoblasts: a novel bridge between oxidative stress and preeclampsia".Placenta.34(12): 1177–1182.doi:10.1016/j.placenta.2013.09.017.PMID24144948.
- ^Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. (December 2012)."Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent".Hepatology.56(6): 2316–2327.doi:10.1002/hep.25938.PMC3493679.PMID22806357.
- ^"Espacenet - Bibliographic data".worldwide.espacenet.com.Retrieved2017-05-04.
- ^"FDA granting Genkyotex Orphan Drug Designation of GKT137831 for IPF - Genkyotex S.A."pauahosting.co.nz.Retrieved2017-05-04.[permanent dead link]
External links[edit]
- NADPH+Oxidaseat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- EC1.6.3.1
