Nerol
Appearance
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
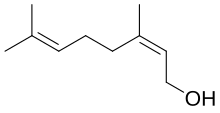
(2Z)-3,7-Dimethylocta-2,6-dien-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.072 |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.25 g/mol |
| Density | 0.881 g/cm3 |
| Boiling point | 224 to 225 °C (435 to 437 °F; 497 to 498 K) at 745 mmHg |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Nerolis amonoterpenoidalcoholfound in manyessential oilssuch aslemongrassandhops.It was originally isolated fromneroli oil,hence its name. This colourless liquid is used in perfumery. Like geraniol, nerol has a sweet rose odor but it is considered to be fresher.[1]Esters and related derivatives of nerol are referred to asneryl,e.g., neryl acetate.
Isomericwith nerol isgeraniol,which istrans- orE-isomer.Nerol readily loses water to form a set of C10 compounds calleddipentene.Nerol can be synthesized bypyrolysisofbeta-pinene,which also affordsmyrcene.Hydrochlorination of myrcene gives a series of isomeric chlorides.
See also
[edit]References
[edit]- ^Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002.doi:10.1002/14356007.a11_141
