Nicotinamide
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌnaɪəˈsɪnəmaɪd/,/ˌnɪkəˈtɪnəmaɪd/ |
| Other names | NAM, 3-pyridinecarboxamide niacinamide nicotinic acid amide vitamin PP nicotinic amide vitamin B3 |
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Routes of administration | oral,topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.002.467 |
| Chemical and physical data | |
| Formula | C6H6N2O |
| Molar mass | 122.127g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.40 g/cm3g/cm3[1] |
| Melting point | 129.5 °C (265.1 °F) |
| Boiling point | 334 °C (633 °F) |
| |
| |
Niacinamideornicotinamideis a form ofvitamin B3found in food and used as adietary supplementand medication.[2][3][4]As a supplement, it is usedorally(swallowed by mouth) to prevent and treatpellagra(niacin deficiency).[3]Whilenicotinic acid(niacin) may be used for this purpose, niacinamide has the benefit of not causingskin flushing.[3]As a cream, it is used to treatacne,and has been observed in clinical studies to improve the appearance of aging skin by reducinghyperpigmentationand redness.[4][5]It is awater-soluble vitamin.Niacinamide is the supplement name, while nicotinamide is the scientific name.
Side effects are minimal.[6][7]At high doses,liver problemsmay occur.[6]Normal amounts are safe for use duringpregnancy.[8]Niacinamide is in thevitamin Bfamily of medications, specifically thevitamin B3complex.[9][10]It is anamideof nicotinic acid.[6]Foods that contain niacinamide includeyeast,meat,milk, andgreen vegetables.[11]
Niacinamide was discovered between 1935 and 1937.[12][13]It is on theWorld Health Organization's List of Essential Medicines.[14][15]Niacinamide is available as ageneric medicationandover the counter.[9]Commercially, niacinamide is made from eithernicotinic acid(niacin) ornicotinonitrile.[13][16]In some countries,grainshave niacinamide added to them.[13]
Medical uses
[edit]Niacin deficiency
[edit]Niacinamide is the preferred treatment forpellagra,caused by niacin deficiency.[3]
Acne
[edit]Niacinamidecreamis used as a treatment foracne.[4]It has anti-inflammatory actions, which may benefit people with inflammatory skin conditions.[17]
Niacinamide increases the biosynthesis ofceramidesin humankeratinocytesin vitro and improves the epidermal permeability barrier in vivo.[18]The application of 2% topical niacinamide for 2 and 4 weeks has been found to be effective in lowering thesebumexcretion rate.[19]Niacinamide has been shown to preventCutibacterium acnes-induced activation oftoll-like receptor 2,which ultimately results in the down-regulation of pro-inflammatoryinterleukin-8production.[20]
Skin cancer
[edit]Niacinamide at doses of 500 to 1000mg a day decreases the risk ofskin cancers,other thanmelanoma,in those at high risk.[21]
Side effects
[edit]Niacinamide has minimal side effects.[6][7]At very high doses above 3g/ day acuteliver toxicityhas been documented in at least one case.[6]Normal doses are safe duringpregnancy.[8]
Chemistry
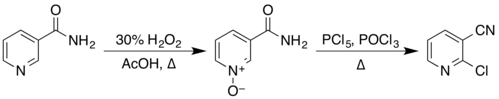
[edit]The structure of nicotinamide consists of apyridinering to which aprimary amidegroup is attached in themetaposition. It is anamideofnicotinic acid.[6]As anaromatic compound,it undergoeselectrophilic substitutionreactions and transformations of its twofunctional groups.Examples of these reactions reported inOrganic Synthesesinclude the preparation of2-chloronicotinonitrileby a two-step process via theN-oxide,[22][23]
fromnicotinonitrileby reaction withphosphorus pentoxide,[24]and from3-aminopyridineby reaction with a solution ofsodium hypobromite,preparedin situfrombromineandsodium hydroxide.[25]

Industrial production
[edit]The hydrolysis ofnicotinonitrileis catalysed by the enzymenitrile hydratasefromRhodococcus rhodochrousJ1,[26][27][16]producing 3500 tons per annum of nicotinamide for use in animal feed.[28]The enzyme allows for a more selective synthesis as further hydrolysis of the amide tonicotinic acidis avoided.[29][30]Nicotinamide can also be made from nicotinic acid. According toUllmann's Encyclopedia of Industrial Chemistry,worldwide 31,000 tons of nicotinamide were sold in 2014.[13]
Biochemistry
[edit]
Nicotinamide, as a part of the cofactornicotinamide adenine dinucleotide(NADH / NAD+) is crucial to life. In cells, nicotinamide is incorporated into NAD+andnicotinamide adenine dinucleotide phosphate(NADP+). NAD+and NADP+arecofactorsin a wide variety of enzymaticoxidation-reductionreactions, most notablyglycolysis,thecitric acid cycle,and theelectron transport chain.[31]If humans ingest nicotinamide, it will likely undergo a series of reactions that transform it into NAD, which can then undergo a transformation to form NADP+.This method of creation of NAD+is called asalvage pathway.However, the human body can produce NAD+from the amino acidtryptophanand niacin without our ingestion of nicotinamide.[32]
NAD+acts as an electron carrier that mediates the interconversion of energy between nutrients and the cell's energy currency,adenosine triphosphate(ATP). In oxidation-reduction reactions, the active part of the cofactor is the nicotinamide. In NAD+,the nitrogen in the aromatic nicotinamide ring is covalently bonded to adenine dinucleotide. The formal charge on the nitrogen is stabilized by the shared electrons of the other carbon atoms in the aromatic ring. When a hydride atom is added onto NAD+to form NADH, the molecule loses its aromaticity, and therefore a good amount of stability. This higher energy product later releases its energy with the release of a hydride, and in the case of the electron transport chain, it assists in formingadenosine triphosphate.[33]
When one mole of NADH is oxidized, 158.2kJ of energy will be released.[33]
Biological role
[edit]Nicotinamide occurs as a component of a variety of biological systems, including within thevitamin Bfamily and specifically thevitamin B3complex.[9][10]It is also a critically important part of the structures ofNADH and NAD+,where theN-substituted aromatic ring in the oxidised NAD+form undergoes reduction with hydride attack to form NADH.[31]TheNADPH/NADP+structures have the same ring, and are involved in similar biochemical reactions.
Nicotinamide can be methylated in the liver to biologically active1-Methylnicotinamidewhen there are sufficient methyl donors.
Food sources
[edit]Niacinamide occurs in trace amounts mainly in meat, fish, nuts, and mushrooms, as well as to a lesser extent in some vegetables.[34]It is commonly added to cereals and other foods. Many multivitamins contain 20–30mg of vitamin B3and it is also available in higher doses.[35]
Compendial status
[edit]Research
[edit]A 2015 trial found niacinamide to reduce the rate of new nonmelanoma skin cancers and actinic keratoses in a group of people at high risk for the conditions.[38]
Niacinamide has been investigated for many additional disorders, including treatment ofbullous pemphigoidnonmelanoma skin cancers.[39]
Niacinamide may be beneficial in treating psoriasis.[40]
There is tentative evidence for a potential role of niacinamide in treating acne, rosacea, autoimmune blistering disorders, ageing skin, and atopic dermatitis.[39]Niacinamide also inhibits poly(ADP-ribose)polymerases(PARP-1), enzymes involved in the rejoining of DNA strand breaks induced by radiation or chemotherapy.[41]ARCON (accelerated radiotherapy plus carbogen inhalation and nicotinamide) has been studied in cancer.[42]
Research has suggested niacinamide may play a role in the treatment ofHIV.[43]
References
[edit]- ^Recordin theGESTIS Substance Databaseof theInstitute for Occupational Safety and Health
- ^Bender DA (2003).Nutritional Biochemistry of the Vitamins.Cambridge University Press.p. 203.ISBN978-1-139-43773-8.Archivedfrom the original on 30 December 2016.
- ^abcdWorld Health Organization(2009). Stuart MC, Kouimtzi M, Hill SR (eds.).WHO Model Formulary 2008.World Health Organization. pp. 496, 500.hdl:10665/44053.ISBN978-92-4-154765-9.
- ^abcBritish National Formulary: BNF 69(69th ed.).British Medical Association.2015. p. 822.ISBN978-0-85711-156-2.
- ^Bissett DL, Oblong JE, Berge CA (July 2005). "Niacinamide: A B vitamin that improves aging facial skin appearance".Dermatologic Surgery.31(7 Pt 2): 860–5, discussion 865.doi:10.1111/j.1524-4725.2005.31732.PMID16029679.
- ^abcdefKnip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, et al. (November 2000)."Safety of high-dose nicotinamide: a review"(PDF).Diabetologia.43(11): 1337–45.doi:10.1007/s001250051536.PMID11126400.S2CID24763480.Archived(PDF)from the original on 22 September 2017.Retrieved20 April2018.
- ^abMacKay D, Hathcock J, Guarneri E (June 2012)."Niacin: chemical forms, bioavailability, and health effects".Nutrition Reviews.70(6): 357–66.doi:10.1111/j.1753-4887.2012.00479.x.PMID22646128.
- ^ab"Niacinamide Use During Pregnancy".Drugs.com.Archivedfrom the original on 30 December 2016.Retrieved29 December2016.
- ^abc"Niacinamide: Indications, Side Effects, Warnings".Drugs.com.6 June 2017.Archivedfrom the original on 5 August 2017.Retrieved30 June2017.
- ^abKrutmann J, Humbert P (2010).Nutrition for Healthy Skin: Strategies for Clinical and Cosmetic Practice.Springer Science & Business Media.p. 153.ISBN978-3-642-12264-4.Archivedfrom the original on 10 April 2017.
- ^Burtis CA, Ashwood ER, Bruns DE (2012).Tietz Textbook of Clinical Chemistry and Molecular Diagnostics(5th ed.).Elsevier Health Sciences.p. 934.ISBN978-1-4557-5942-2.Archivedfrom the original on 30 December 2016.
- ^Sneader W (2005).Drug Discovery: A History.John Wiley & Sons.p. 231.ISBN978-0-470-01552-0.Archivedfrom the original on 30 December 2016.
- ^abcdBlum R (2015). "Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide)".Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide.Ullmann's Encyclopedia of Industrial Chemistry(6th ed.). Weinheim:Wiley-VCH.pp. 1–9.doi:10.1002/14356007.o27_o14.pub2.ISBN978-3-527-30385-4.
- ^World Health Organization(2019).World Health Organization model list of essential medicines: 21st list 2019.Geneva: World Health Organization.hdl:10665/325771.WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^World Health Organization(2021).World Health Organization model list of essential medicines: 22nd list (2021).Geneva: World Health Organization.hdl:10665/345533.WHO/MHP/HPS/EML/2021.02.
- ^abSchmidberger JW, Hepworth LJ, Green AP, Flitsch SL (2015)."Enzymatic Synthesis of Amides".In Faber K, Fessner WD, Turner NJ (eds.).Biocatalysis in Organic Synthesis 1.Science of Synthesis.Georg Thieme Verlag.pp. 329–372.ISBN978-3-13-176611-3.Archivedfrom the original on 5 November 2017.
- ^Niren NM (January 2006). "Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review".Cutis.77(1 Suppl): 11–6.PMID16871774.
- ^Tanno O, Ota Y, Kitamura N, Katsube T, Inoue S (September 2000). "Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier".The British Journal of Dermatology.143(3): 524–31.doi:10.1111/j.1365-2133.2000.03705.x.PMID10971324.S2CID21874670.
- ^Draelos ZD, Matsubara A, Smiles K (June 2006). "The effect of 2% niacinamide on facial sebum production".Journal of Cosmetic and Laser Therapy.8(2): 96–101.doi:10.1080/14764170600717704.PMID16766489.S2CID36713665.
- ^Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. (August 2002)."Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses".Journal of Immunology.169(3): 1535–41.doi:10.4049/jimmunol.169.3.1535.PMC4636337.PMID12133981.
- ^Snaidr VA, Damian DL, Halliday GM (February 2019)."Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety".Experimental Dermatology.28(Suppl 1): 15–22.doi:10.1111/exd.13819.PMID30698874.
- ^Taylor EC, Crovetti AJ (1957)."Nicotinamide-1-oxide".Organic Syntheses.37:63.doi:10.15227/orgsyn.037.0063;Collected Volumes,vol. 4, p. 704.
- ^Taylor EC, Crovetti AJ (1957)."2-Chloronicitinonitrile".Organic Syntheses.37:12.doi:10.15227/orgsyn.037.0012;Collected Volumes,vol. 4, p. 166.
- ^Teague PC, Short WA (1953)."Nicotinonitrile".Organic Syntheses.33:52.doi:10.15227/orgsyn.033.0052;Collected Volumes,vol. 4, p. 706.
- ^Allen CF, Wolf CN (1950)."3-Aminopyridine".Organic Syntheses.30:3.doi:10.15227/orgsyn.030.0003;Collected Volumes,vol. 4, p. 45.
- ^Nagasawa T, Mathew CD, Mauger J, Yamada H (July 1988)."Nitrile Hydratase-Catalyzed Production of Nicotinamide from 3-Cyanopyridine in Rhodococcus rhodochrous J1".Applied and Environmental Microbiology.54(7): 1766–1769.Bibcode:1988ApEnM..54.1766N.doi:10.1128/AEM.54.7.1766-1769.1988.PMC202743.PMID16347686.
- ^Hilterhaus L, Liese A (2007)."Building Blocks".In Ulber R, Sell D (eds.).White Biotechnology.Advances in Biochemical Engineering / Biotechnology. Vol. 105.Springer Science & Business Media.pp. 133–173.doi:10.1007/10_033.ISBN978-3-540-45695-7.PMID17408083.S2CID34552222.Archivedfrom the original on 5 November 2017.
- ^Asano Y (2015)."Hydrolysis of Nitriles to Amides".In Faber K, Fessner WD, Turner NJ (eds.).Biocatalysis in Organic Synthesis 1.Science of Synthesis.Georg Thieme Verlag.pp. 255–276.ISBN978-3-13-176611-3.Archivedfrom the original on 5 November 2017.
- ^Petersen M, Kiener A (1999). "Biocatalysis".Green Chem.1(2): 99–106.doi:10.1039/A809538H.
- ^Servi S, Tessaro D, Hollmann F (2015)."Historical Perspectives: Paving the Way for the Future".In Faber NJ, Fessner WD, Turner K (eds.).Biocatalysis in Organic Synthesis 1.Science of Synthesis.Georg Thieme Verlag.pp. 1–39.ISBN978-3-13-176611-3.Archivedfrom the original on 5 November 2017.
- ^abBelenky P, Bogan KL, Brenner C (January 2007)."NAD+ metabolism in health and disease"(PDF).Trends in Biochemical Sciences.32(1): 12–9.doi:10.1016/j.tibs.2006.11.006.PMID17161604.Archived(PDF)from the original on 27 September 2007.
- ^Williams AC, Cartwright LS, Ramsden DB (March 2005)."Parkinson's disease: the first common neurological disease due to auto-intoxication?".QJM.98(3): 215–26.doi:10.1093/qjmed/hci027.PMID15728403.
- ^abCasiday R, Herman C, Frey R (5 September 2008)."Energy for the Body: Oxidative Phosphorylation".www.chemistry.wustl.edu.Department of Chemistry,Washington University in St. Louis.Archivedfrom the original on 22 November 2016.Retrieved14 March2017.
- ^Rolfe HM (December 2014). "A review of nicotinamide: treatment of skin diseases and potential side effects".Journal of Cosmetic Dermatology.13(4): 324–8.doi:10.1111/jocd.12119.PMID25399625.S2CID28160151.
- ^Ranaweera A (2017)."Nicotinamide".DermNet New Zealand (www.dermnetnz.org).DermNet New Zealand Trust.Archivedfrom the original on 25 March 2017.Retrieved30 June2017.
- ^British Pharmacopoeia Commission Secretariat (2009).Index, BP 2009(PDF).Archived fromthe original(PDF)on 22 July 2011.Retrieved4 February2010.
- ^Japanese Pharmacopoeia(PDF)(15th ed.). 2006. Archived fromthe original(PDF)on 22 July 2011.Retrieved4 February2010.
- ^Minocha R, Damian DL, Halliday GM (January 2018)."Melanoma and nonmelanoma skin cancer chemoprevention: Arole for nicotinamide? ".Photodermatology, Photoimmunology & Photomedicine.34(1): 5–12.doi:10.1111/phpp.12328.PMID28681504.
- ^abChen AC, Damian DL (August 2014). "Nicotinamide and the skin".The Australasian Journal of Dermatology.55(3): 169–75.doi:10.1111/ajd.12163.PMID24635573.S2CID45745255.
- ^Namazi MR (August 2003)."Nicotinamide: a potential addition to the anti-psoriatic weaponry".FASEB Journal.17(11): 1377–9.doi:10.1096/fj.03-0002hyp.PMID12890690.S2CID39752891.
- ^"Definition of niacinamide".NCI Drug Dictionary.National Cancer Institute.2 February 2011.Archivedfrom the original on 28 April 2015.Retrieved30 June2017.
- ^Kaanders JH, Bussink J, van der Kogel AJ (December 2002). "ARCON: a novel biology-based approach in radiotherapy".The Lancet. Oncology.3(12): 728–37.doi:10.1016/s1470-2045(02)00929-4.PMID12473514.
- ^Mandavilli A (7 July 2020)."Patient Is Reported Free of H.I.V., but Scientists Urge Caution".The New York Times.Archivedfrom the original on 23 September 2020.Retrieved22 September2020.