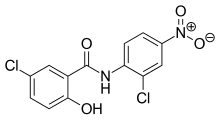
Niclosamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Niclocide, Fenasal, Phenasal, others[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.000.052 |
| Chemical and physical data | |
| Formula | C13H8Cl2N2O4 |
| Molar mass | 327.12g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 225 to 230 °C (437 to 446 °F) |
| |
| |
Niclosamide,sold under the brand nameNiclocideamong others, is ananthelminticmedication used to treattapeworminfestations, includingdiphyllobothriasis,hymenolepiasis,andtaeniasis.It is not effective against other worms such asflukesorroundworms.[2]It is taken by mouth.[3]
Side effects include nausea, vomiting, abdominal pain, and itchiness. It may be used during pregnancy.[3]It works by blockingglucose uptakeandoxidative phosphorylationby the worm.[4]
Niclosamide was first synthesized in 1958.[5]It is on theWorld Health Organization's List of Essential Medicines.[6]Niclosamide is not available for human use in the United States.[7]
Side effects[edit]
Side effects include nausea, vomiting, abdominal pain,constipation,and itchiness.[3]Rarely, dizziness, skin rash, drowsiness, perianal itching, or an unpleasant taste occur. For some of these reasons,praziquantelis a preferable and equally effective treatment for tapeworm infestation.[citation needed] Important Note: Niclosamide kills the pork tapeworm and results in its digestion. This then may cause a multitude of viable eggs to be released and may result incysticercosis.Therefore, a purge should be given 1 or two hours after treatment. CNS cysticercosis is a life-threatening condition and may require brain surgery.[8][9]
Mechanism of action[edit]
Niclosamide inhibitsglucoseuptake, oxidativephosphorylation,and anaerobic metabolism in the tapeworm.[10]
Other applications[edit]
Niclosamide's metabolic effects are relevant to a wide ranges of organisms, and accordingly it has been applied as a control measure to organisms other than tapeworms. For example, it is an active ingredient in some formulations such as Bayluscide for killinglampreylarvae,[11][12]as a molluscide,[13]and as a general purpose piscicide in aquaculture. Niclosamide has a short half-life in water in field conditions; this makes it valuable in ridding commercial fish ponds of unwanted fish; it loses its activity soon enough to permit re-stocking within a few days of eradicating the previous population.[13]Researchers have found that niclosamide is effective in killing invasivezebra musselsin cool waters.[14]
Research[edit]
Niclosamide is under investigation for applications against types of cancer,[15]bacterial infections,[16]or viral infections.[17][18]
In 2018, niclosamide was observed to be a potent activator ofPTEN-induced kinase 1in primary cortical neurons.[19]
References[edit]
- ^CID 4477fromPubChem
- ^"Niclosamide Advanced Patient Information - Drugs.com".www.drugs.com.Archivedfrom the original on 20 December 2016.Retrieved8 December2016.
- ^abcWorld Health Organization(2009). Stuart MC, Kouimtzi M, Hill SR (eds.).WHO Model Formulary 2008.World Health Organization. pp. 81, 87, 591.hdl:10665/44053.ISBN9789241547659.
- ^Lanusse CE, Alvarez LI, Sallovitz JM, Mottier ML, Sanchez Bruni SF (13 May 2013)."Antinematodal Drugs".In Riviere JE, Papich MG (eds.).Veterinary Pharmacology and Therapeutics.John Wiley & Sons. p. 1096.ISBN978-1-118-68590-7.Archivedfrom the original on 10 September 2017.
- ^Mehlhorn H (2008).Encyclopedia of Parasitology: A-M.Springer Science & Business Media. p. 483.ISBN978-3-540-48994-8.Archivedfrom the original on 2016-12-20.
- ^World Health Organization(2019).World Health Organization model list of essential medicines: 21st list 2019.Geneva: World Health Organization.hdl:10665/325771.WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^"Dipylidium - Resources for Health Professionals".U.S. Centers for Disease Control and Prevention (CDC).21 May 2020.
- ^Remington JP, Osol A, eds. (1980).Remington's Pharmaceutical Sciences(16th ed.). Easton: Mack Pub. Co. p. 1182.OCLC925174053.
- ^The Merck Manual of Diagnosis and Therapy(14th ed.). Rahway: Merck Sharp & Dohme Research Laboratories. 1982. p. 176.ISBN978-0-911910-03-2.
- ^Weinbach EC, Garbus J (March 1969). "Mechanism of action of reagents that uncouple oxidative phosphorylation".Nature.221(5185): 1016–1018.Bibcode:1969Natur.221.1016W.doi:10.1038/2211016a0.PMID4180173.S2CID4209497.
- ^Boogaard, Michael A. Delivery Systems of Piscicideshttps://www.usbr.gov/lc/phoenix/biology/azfish/pdf/borfinalreport6t7.pdf.Archived(PDF)from the original on 2017-06-01.Retrieved2017-05-30.
{{cite web}}:Missing or empty|title=(help) - ^Verdel K.Dawson (2003). "Environmental Fate and Effects of the Lampricide Bayluscide: a Review".Journal of Great Lakes Research.29(Supplement 1): 475–492.Bibcode:2003JGLR...29..475D.doi:10.1016/S0380-1330(03)70509-7.
- ^ab"WHO Specifications And Evaluations. For Public Health Pesticides. Niclosamide"(PDF).Archived fromthe original(PDF)on 2017-01-10.Retrieved2019-08-07.
- ^Blank L (30 October 2018)."Researchers find new methods to combat invasive zebra mussels".The Minnesota Daily.Archived fromthe originalon 2018-11-21.Retrieved2018-11-19.
- ^"Clinical Trials Using Niclosamide".NCI.Retrieved20 March2019.
- ^Rajamuthiah R, Fuchs BB, Conery AL, Kim W, Jayamani E, Kwon B, et al. (April 2015). Planet PJ (ed.)."Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus".PLOS ONE.10(4): e0124595.Bibcode:2015PLoSO..1024595R.doi:10.1371/journal.pone.0124595.PMC4405337.PMID25897961.
- ^Li Y, Li P, He Q, Zhang R, Li Y, Kamar N, et al. (January 2022)."Niclosamide inhibits hepatitis E virus through suppression of NF-kappaB signalling".Antiviral Research.197:105228.doi:10.1016/j.antiviral.2021.105228.PMID34929248.
- ^Braga L, Ali H, Secco I, Chiavacci E, Neves G, Goldhill D, et al. (June 2021)."Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia".Nature.594(7861): 88–93.Bibcode:2021Natur.594...88B.doi:10.1038/s41586-021-03491-6.PMC7611055.PMID33827113.
- ^Barini E, Miccoli A, Tinarelli F, Mulholland K, Kadri H, Khanim F, et al. (March 2018)."The Anthelmintic Drug Niclosamide and Its Analogues Activate the Parkinson's Disease Associated Protein Kinase PINK1".ChemBioChem.19(5): 425–429.doi:10.1002/cbic.201700500.PMC5901409.PMID29226533.
Further reading[edit]
- Taber CW, Venes D, Thomas CL (2001).Taber's Cyclopedic Medical Cictionary(19th ed.). Philadelphia: F.A.Davis Co.ISBN978-0-8036-0655-5.
- World Health Organization (1995)."Helminths: Cestode (tapeworm) infection: Niclosamide".WHO model prescribing information: drugs used in parasitic diseases(2nd ed.).World Health Organization(WHO).hdl:10665/41765.ISBN9789241401043.
External links[edit]
- "Niclosamide".Drug Information Portal.U.S. National Library of Medicine.
- Niclosamidein the Pesticide Properties DataBase (PPDB)
- "MedlinePlus Drug Information: Niclosamide (Oral)".MedlinePlus.U.S. National Library of Medicine. 1995-06-23. Archived fromthe originalon 2006-12-16.
