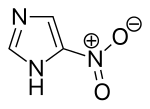
Nitroimidazole
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Nitro-1H-imidazole | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.296 |
| EC Number |
|
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C3H3N3O2 | |
| Molar mass | 113.076g·mol−1 |
| Melting point | 303 °C (577 °F; 576 K) (decomposes) |
| Hazards | |
| GHSlabelling: | |

| |
| Warning | |
| H302 | |
| P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P312,P304+P340,P305+P351+P338,P312,P321,P322,P330,P332+P313,P337+P313,P362,P363,P403+P233,P405,P501 | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Nitroimidazolesare the group oforganic compoundsconsisting of animidazolering with at least onenitro groupsubstituent. The term also refers to the class of antibiotics that have nitroimidazole in their structures.[2]These antibiotics commonly include the 5-nitroimidazolepositional isomer.
Synthesis[edit]

Imidazoleundergoes anitrationreaction with a mixture ofnitric acidandsulfuric acidto give 5-nitroimidazole.
Nitroimidazole antibiotics[edit]

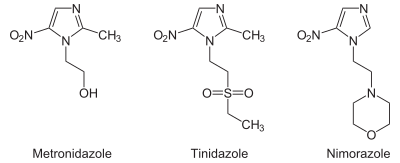
From the chemistry perspective, nitroimidazole antibiotics can be classified according to the location of the nitrofunctional group.Structures with names 4- and 5-nitroimidazole are equivalent from the perspective of drugs since these tautomers readily interconvert. Drugs of the 5-nitro variety includemetronidazole,tinidazole,nimorazole,dimetridazole,pretomanid,ornidazole,megazol,andazanidazole.Drugs based on 2-nitroimidazoles includebenznidazoleandazomycin.[3]
Nitroimidazole antibiotics have been used to combatanaerobicbacterialandparasiticinfections.[4]Perhaps the most common example ismetronidazole.Other heterocycles such as nitrothiazoles (thiazole) are also used for this purpose. Nitroheterocycles may bereductivelyactivated inhypoxiccells, and then undergoredoxrecycling ordecomposeto toxic products.[5]
References[edit]
- ^4-NitroimidazoleatSigma-Aldrich
- ^Edwards, David I. (1993). "Nitroimidazole drugs-action and resistance mechanisms I. Mechanism of action".Journal of Antimicrobial Chemotherapy.31(1): 9–20.doi:10.1093/jac/31.1.9.PMID8444678.
- ^Jenks, Peter J. (2010-01-01), Finch, Roger G.; Greenwood, David; Norrby, S. Ragnar; Whitley, Richard J. (eds.),"CHAPTER 24 - Nitroimidazoles",Antibiotic and Chemotherapy (Ninth Edition),London: W.B. Saunders, pp. 292–300,doi:10.1016/b978-0-7020-4064-1.00024-5,ISBN978-0-7020-4064-1,retrieved2023-10-18
- ^Mital A (2009)."Synthetic Nitroimidazoles: Biological Activities and Mutagenicity Relationships".Sci Pharm.77(3): 497–520.doi:10.3797/scipharm.0907-14.
- ^Juchau, MR (1989). "Bioactivation in chemical teratogenesis".Annu. Rev. Pharmacol. Toxicol.29:165–167.doi:10.1146/annurev.pa.29.040189.001121.PMID2658769.
