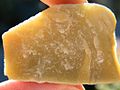
Serpentine subgroup
| Serpentine | |
|---|---|
 | |
| General | |
| Category | Phyllosilicates |
| Formula (repeating unit) | X3Si2O5(OH)4, with X = Mg2+,Fe2+,Ni2+,Mn2+,Zn2+ |
| IMA symbol | Srp |
| Crystal system | Monoclinic |
| Identification | |
| Color | Green, yellowish-green, blueish-gray (antigorite) Green, brown, light yellow to white (lizardite) Greyish green to white (chrysotile) |
| Cleavage | Almost perfect |
| Fracture | Brittle |
| Mohs scalehardness | 2.5–6 (original) 3.5–4.0 (antigorite) 2.5 (lizardite) 2.5–3.0 (chrysotile) |
| Luster | Vitreous, silky, greasy, waxy |
| Streak | White,greenish-white |
| Specific gravity | 2.2–2.9 |
| Optical properties | Biaxial (–) |
| Refractive index | 1.538–1.57 (Tolerance:0.004/–0.07) |
| Birefringence | 0.005–0.012 |
| Ultravioletfluorescence | SWUV: inert to weak blue; LWUV: inert to weak green |
| Diagnostic features | Color, cleavage |
| References | [1][2][3][4] |

Serpentine subgroup(part of thekaolinite-serpentine group in the category of phyllosilicates)[1]are greenish, brownish, or spotted minerals commonly found inserpentinite.They are used as a source ofmagnesiumandasbestos,and as decorative stone.[5]The name comes from the greenish color and smooth or scaly appearance from theLatinserpentinus,meaning "snake-like".
Serpentine subgroup is a set of commonrock-forminghydrousmagnesiumironphyllosilicate((Mg,Fe)
3Si
2O
5(OH)
4)minerals,resulting from themetamorphismof the minerals that are contained inmafictoultramafic rocks.[6]They may contain minor amounts of other elements includingchromium,manganese,cobaltornickel.Inmineralogyandgemology,serpentine may refer to any of the 20 varieties belonging to the serpentine subgroup. Owing to admixture, these varieties are not always easy to individualize, and distinctions are not usually made. There are three important mineralpolymorphsof serpentine:antigorite,lizarditeandchrysotile.
Serpentine minerals arepolymorphous,meaning that they have the samechemical formulae,but the atoms are arranged into different structures, orcrystal lattices.[7]Chrysotile,which has afibrous habit,is one polymorph of serpentine and is one of the more importantasbestosminerals. Other polymorphs in the serpentine subgroup may have aplaty habit.Antigoriteandlizarditeare the polymorphs with platy habit.
Many types of serpentine have been used forjewelryandhardstone carving,sometimes under the name "false jade" or "Teton jade".[8][9]
Properties and structure
[edit]
Most serpentines are opaque to translucent, light (specific gravitybetween 2.2 and 2.9), soft (hardness2.5–4), infusible and susceptible toacids.[1]All aremicrocrystallineand massive inhabit,never being found as singlecrystals.Lustremay be vitreous, silky or greasy. Colors range from white to grey, yellow to green, and brown to black, and are often splotchy or veined. Many are intergrown with other minerals, such ascalciteanddolomite.
The basic structural unit of serpentine is apolar layer0.72 nm thick. AMg-richtrioctahedral sheetis tightly linked on one side to a singletetrahedral silicate sheet,regardless of the 3–5% larger lateral lattice dimensions of theoctahedral sheet.[10]The second level of the structure organized into different serpentine species originates partly to compensate the intra-layerstressdue to this dimensional misfit. Good compensation results in a nearly constant layercurvature,with the larger octahedral sheet on the convex side. However, such curvature weakens theH-bondingbetween the layers. H-bonding tries to maintain flat layers, but this competes with the requirements of misfit compensation. As a result, the layers are locally either curved or flat.[11]Antigorite,lizarditeandchrysotilehave the samechemical composition,but their different layer of curvatures result in lamellar agglomerated antigorite and lizardite and fibrous chrysotile elongatedmineralparticles.[12][13]
Occurrence
[edit]Serpentine minerals are ubiquitous in many geological systems where hydrothermal alteration of ultramafic rocks is possible, in both terrestrial (oceanic hydrothermalism, subduction zones and transform faulting) and extraterrestrial environments.[14]The process of alteration frommaficminerals to serpentine group minerals is calledserpentinization.Serpentine minerals are often formed by the hydration ofolivine-rich ultramafic rocks at relatively low temperatures (0 to ~600 °C).[15]The chemical reaction turns olivine into serpentine minerals. They may also have their origins inmetamorphicalterations ofperidotiteandpyroxene.Serpentines may alsopseudomorphouslyreplace other magnesium silicates. Incomplete alteration causes the physical properties of serpentines to vary widely.
Antigorite is thepolymorphof serpentine that most commonly forms during metamorphism of wet ultramafic rocks and is stable at the highest temperatures—to over 600 °C (1,100 °F) at depths of 60 km (37 mi) or so. In contrast, lizardite and chrysotile typically form near the Earth's surface and break down at relatively low temperatures, probably well below 400 °C (800 °F). It has been suggested that chrysotile is never stable relative to either of the other two serpentine polymorphs.
Samples of the oceanic crust and uppermost mantle from ocean basins document thatultramaficrocks there commonly contain abundant serpentine. Antigorite contains water in its structure, about 13 percent by weight. Hence, antigorite may play an important role in the transport of water into the earth insubductionzones and in the subsequent release of water to create magmas inisland arcs,and some of the water may be carried to yet greater depths.
Occurrence is worldwide, notable localities includeNew Caledonia,Canada(Quebec),US(northernCalifornia,Rhode Island,Connecticut,Massachusetts,Marylandand southernPennsylvania),[16]Afghanistan,Britain(theLizardpeninsula inCornwall),Ireland,Greece(Thessaly),China,Russia(Ural Mountains),France,Korea,Austria(StyriaandCarinthia),India(Assam,andManipur),Myanmar(Burma),New Zealand,NorwayandItaly.
Uses
[edit]

Serpentines find use in industry for several purposes, such as railway ballasts, building materials, and the asbestiform types find use as thermal and electrical insulation (chrysotileasbestos). The asbestos content can be released into the air when serpentine is excavated and if it is used as a road surface, forming a long-term health hazard by breathing. Asbestos from serpentine can also appear at low levels in water supplies through normal weathering processes, but there is as yet no fully proven health hazard associated with use or ingestion, although the EPA states an increased risk of developing benign intestinal polyps can occur.[17]In its natural state, some forms of serpentine react with carbon dioxide and re-release oxygen into the atmosphere.
The more attractive and durable varieties (all of the antigorite) are termed "noble" or "precious" serpentine and are used extensively asgemsand inornamental carvings.The town ofBherain the historicPunjab provinceof theIndian subcontinentwas known for centuries for finishing a relatively pure form of green serpentine obtained from quarries inAfghanistanintolapidarywork, cups, ornamental sword hilts, and dagger handles.[9]This high-grade serpentine ore was known assang-i-yashminPersian,or 'false jade' in English, and was used for generations by Indian craftsmen for lapidary work.[9][18]It is easily carved, taking a good polish, and is said to have a pleasingly greasy feel.[19]Less valuable serpentine ores of varying hardness and clarity are also sometimes dyed to imitatejade.[19]Misleading synonyms for this material include "Suzhou jade", "Styrian jade", and "New jade".
New Caledonian serpentine is particularly rich in nickel. TheMāoriofNew Zealandonce carved beautiful objects from local serpentine, which they calledtangiwai,meaning "tears".
Thelapis atraciusof theRomans,now known asverde antique,or verde antic, is a serpentinitebrecciapopular as a decorative facing stone. In classical times it was mined atCasambala,Thessaly,Greece.Serpentinitemarblesare also widely used: GreenConnemara marble(or 'Irish green marble') fromConnemara,Ireland(and many other sources[citation needed]), and redRosso di Levanto marblefrom Italy. Use is limited to indoor settings as serpentinites do notweatherwell.
Potential harm
[edit]Soils derived from serpentine are toxic to manyplants,because of high levels ofnickel,chromium,andcobalt;growth of many plants is also inhibited by low levels ofpotassiumandphosphorusand a low ratio ofcalcium/magnesium.Theflorais generally very distinctive, with specialized, slow-growing species. Areas ofserpentine-derived soilwill show as strips ofshrublandand open, scattered smalltrees(oftenconifers) within otherwiseforestedareas; these areas are calledserpentine barrens.
Antigorite variety
[edit]
Lamellated antigorite occurs in tough, pleated masses. It is usually dark green, but may also be yellowish, gray, brown or black. It has a hardness of 3.5–4 and its luster is greasy. The monoclinic crystals show micaceouscleavageand fuse with difficulty. Antigorite is named after its type locality, the Geisspfad serpentinite,Valle Antigorioin the border region ofItaly/Switzerland.
Bowenite
[edit]Bowenite,a variety of antigorite, is an especially hard serpentine (5.5) of light to dark apple green color, often mottled with cloudy white patches and darker veining. It is the serpentine most frequently encountered in carving and jewelry. The name 'retinalite' is sometimes applied to yellow bowenite. The New Zealand material is calledtangiwai.
Although not an official species, bowenite is the state mineral ofRhode Island,United States: this is also the variety's type locality. A bowenitecabochonfeatured as part of the "Our Mineral Heritage Brooch", was presented to U.S. First Lady Mrs.Lady Bird Johnsonin 1967.
Williamsite is an American local varietal name for antigorite that is oil-green with black crystals ofchromiteormagnetiteoften included. Somewhat resembling fine jade, williamsite is cut into cabochons and beads. It is found mainly inMarylandandPennsylvania.[20]
Gymnite
[edit]Gymnite is an amorphous form of antigorite.[21]It was originally found in theBare HillsofMaryland,and is named from theGreek,'gymnos',meaning "bare" or "naked".
State emblem
[edit]In 1965, theCaliforniaLegislature designated the mineral serpentine as "the official State Rock and lithologic emblem".[22]
Gallery
[edit]-
Serpentinite from the Precambrian of Michigan, US
-
Serpentinite from East Dover Ultramafic Body, Ordovician; roadcut east of East Dover, Vermont, US
-
Antigorite from Clay Geo, Unst, Shetland Islands, Scotland, UK
-
Antigorite from Lord Brassey Mine, Heazlewood district, Tasmania, Australia
-
Slab of curiously patterned antigorite from the Jeffrey Mine, Quebec, Canada
-
Picrolite (antigorite) from Quebec looking in color and form like a bit of celery
-
Genthite (antigorite) from Wood's Chrome Mine. The bright green, lustrous antigorite richly covering this specimen has an unusual knobby/bubbly/drusy form.
-
Bowenite (Antigorite) from Asbestos mine, Thurman Township, Warren County, New York, US
-
Polished slab of bowenite serpentine, a variety of antigorite. Typical cloudy patches and veining are apparent.
-
Turban ornament, North India, Delhi or Jaipur, 18th–19th century, antigorite, gold, pearls, glass. Ethnological Museum, Berlin
-
Serpentine that has turned green, 17th century
-
College Hall atUniversity of Pennsylvania
-
Old Library Building atWest Chester University
-
Recitation Hall atWest Chester University
References
[edit]- ^abc"Serpentine Subgroup".mindat.org.Retrieved30 April2021.
- ^"pyrophyllite | mineral | Britannica".www.britannica.com.Retrieved2022-11-15.
- ^"Serpentine | NOVA Mineralogy".Retrieved2022-11-15.
- ^"Serpentine: The mineral Serpentine information and pictures".www.minerals.net.Retrieved2022-11-15.
- ^Serpentine, American Heritage Dictionary
- ^"Serpentine definition in the Dictionary of Geology".Retrieved9 July2018.
- ^"Serpentine: The mineral Serpentine information and pictures".www.minerals.net.Retrieved4 April2018.
- ^National Park ServiceArchived2010-09-30 at theWayback Machine
- ^abcHunter, Sir William Wilson and Burn, Sir Richard, The Imperial Gazetteer of India, Vol. 3, Oxford, England: Clarendon Press, Henry Frowde Publishers (1907), p. 242
- ^F. J. Wicks; E. J. W. Whittaker (August 1, 1975)."A reappraisal of the structures of the serpentine minerals".The Canadian Mineralogist.13(3): 227–243.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Bernard W. Evans, Keiko Hattori, and Alain Baronnet (April 1, 2013)."Serpentinite: What, Why, Where?".Elements.9(2): 99–106.Bibcode:2013Eleme...9...99E.doi:10.2113/gselements.9.2.99.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^D. Hršak, G. Sučik, L. Lazić (2008)."The thermophysical properties of serpentinite".Metalurgija.47(1).
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Shiwei Zhou, Yonggang Wei, Bo Li, Baozhong Ma, Chengyan Wang, Hua Wang (August 5, 2017)."Kinetics study on the dehydroxylation and phase transformation of Mg3Si2O5(OH)4".Journal of Alloys and Compounds.713:180–186.doi:10.1016/j.jallcom.2017.04.162.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^J. F. Mustard, F. Poulet, A. Gendrin, J.-P. Bibring, Y. Lagevin, B. Gondet, N. Mangold, G. Bellucci, And F. Altieri (March 11, 2005)."Olivine and Pyroxene Diversity in the Crust of Mars".Science.307(5715): 1594–1597.Bibcode:2005Sci...307.1594M.doi:10.1126/science.1109098.PMID15718427.S2CID15548016.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Evans, Bernard W. (2004-06-01)."The Serpentinite Multisystem Revisited: Chrysotile Is Metastable".International Geology Review.46(6): 479–506.Bibcode:2004IGRv...46..479E.doi:10.2747/0020-6814.46.6.479.ISSN0020-6814.S2CID98271088.
- ^"Slate – The Delta Story: A Heritage To Be Preserved".JONES, Jeri L., presented to the Geological Society of America's Northeastern Section.March 2005. Archived fromthe originalon July 14, 2011.RetrievedJune 3,2010.
- ^"National Primary Drinking Water Regulations".30 November 2015.
- ^Watt, Sir George,The Commercial Products of India,London: John Murray Publishers (1908), p. 561
- ^abThe Stone Age Jewels: Serpentine,retrieved 2 October 2011[permanent dead link]
- ^http://www.cst.cmich.edu/users/dietr1rv/serpentine.htmArchived2004-06-22 at theWayback MachineGemrocks, R. V. Dietrich, 2005
- ^"Gymnite: Gymnite mineral information and data".www.mindat.org.Retrieved4 April2018.
- ^California Government Code § 425.2;see"CA Codes (Gov:420-429.8)".Archived fromthe originalon 2009-06-28.Retrieved2009-12-24.
External links
[edit]- Mineral description from Mineral galleries
- Evans, Bernard W. (2004).The Serpentinite Multisystem Revisited: Chrysotile is Metastable.In: International Geology Review, v. 46, pages 479–506
- Myron G. (2003).Igneous and Metamorphic Petrology, 2nd edition.Blackwell Publishing.ISBN1-4051-0588-7
- Kruckeberg, Arthur R. (2002).Geology and Plant Life: the Effects of Landforms and Rock Types on Plants.Seattle: University of Washington Press.ISBN0-295-98452-X