PPADS
Appearance
| |
| Names | |
|---|---|
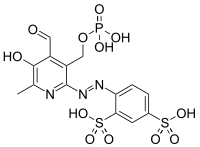
| IUPAC name
4-[(E)-{4-formyl-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]pyridin-2-yl}diazenyl]benzene-1,3-disulfonic acid
| |
| Other names
PPADS
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChemCID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H14N3O12PS2 | |
| Molar mass | 511.37g·mol−1 |
| Appearance | Orange solid |
| 100 mM (tetrasodium salt)[1] | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
PPADS(pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid) is a selective purinergicP2Xantagonist.[2]It is able to block contractions of rabbit vas deferens induced byATPor α,β,methylene-ATP. It appears to be relatively selective for P2X receptors, having no appreciable activity atα1adrenergic,muscarinicM2andM3,histamineH1,and adenosineA1receptors.[3]
References
[edit]- ^PPADS tetrasodium salt,Santa Cruz Biotechnology
- ^Ziganshin, AU (December 1993)."PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder".British Journal of Pharmacology.110(4): 1491–95.doi:10.1111/j.1476-5381.1993.tb13990.x.PMC2175839.PMID8306091.
- ^Lambrecht, G. (1992). "PPADS, a novel functionally selective antagonist of P2 purinoreceptor mediated responses".European Journal of Pharmacology.217(2–3): 217–19.doi:10.1016/0014-2999(92)90877-7.PMID1330591.
