Pentose
Inchemistry,apentoseis amonosaccharide(simple sugar) with fivecarbonatoms.[1]Thechemical formulaof many pentoses isC
5H
10O
5,and theirmolecular weightis 150.13 g/mol.[2]
Pentoses are very important inbiochemistry.Riboseis a constituent ofRNA,and the related molecule,deoxyribose,is a constituent ofDNA.Phosphorylatedpentoses are important products of thepentose phosphate pathway,most importantlyribose 5-phosphate(R5P), which is used in the synthesis ofnucleotidesandnucleic acids,anderythrose 4-phosphate(E4P), which is used in the synthesis of aromaticamino acids.
Like some other monosaccharides, pentoses exist in two forms, open-chain (linear) or closed-chain (cyclic), that easily convert into each other in water solutions.[3]The linear form of a pentose, which usually exists only in solutions, has an open-chain backbone of five carbons. Four of these carbons have onehydroxylfunctional group(–OH) each, connected by a singlebond,and one has an oxygen atom connected by a double bond (=O), forming acarbonylgroup (C=O). The remaining bonds of the carbon atoms are satisfied by sixhydrogenatoms. Thus the structure of the linear form is H–(CHOH)x–C(=O)–(CHOH)4-x–H, wherexis 0, 1, or 2.
The term "pentose" sometimes is assumed to includedeoxypentoses,such asdeoxyribose:compounds with general formulaC
5H
10O
5-ythat can be described as derived from pentoses by replacement of one or more hydroxyl groups with hydrogen atoms.
Classification
[edit]Thealdopentosesare a subclass of the pentoses which, in the linear form, have the carbonyl at carbon 1, forming analdehydederivative with structure H–C(=O)–(CHOH)4–H. The most important example isribose.Theketopentosesinstead have the carbonyl at positions 2 or 3, forming aketonederivative with structure H–CHOH–C(=O)–(CHOH)3–H (2-ketopentose) or H–(CHOH)2–C(=O)–(CHOH)2–H (3-ketopentose). The latter is not known to occur in nature and are difficult to synthesize.
In the open form, there are eight aldopentoses and four 2-ketopentoses,stereoisomersthat differ in the spatial position of the hydroxyl groups. These forms occur in pairs ofoptical isomers,generally labelled "D"or"L"by conventional rules (independently of theiroptical activity).
Aldopentoses
[edit]The aldopentoses have threechiral centers;therefore, eight (23) differentstereoisomersare possible.
 D-Arabinose |
 D-Lyxose |
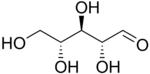 D-Ribose |
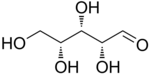 D-Xylose |
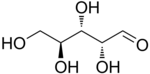 L-Arabinose |
 L-Lyxose |
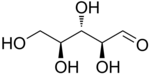 L-Ribose |
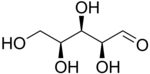 L-Xylose |
Riboseis a constituent ofRNA,and the related molecule,deoxyribose,is a constituent ofDNA.Phosphorylated pentoses are important products of thepentose phosphate pathway,most importantlyribose 5-phosphate(R5P), which is used in the synthesis ofnucleotidesand nucleic acids, anderythrose 4-phosphate(E4P), which is used in the synthesis ofaromatic amino acids.
Ketopentoses
[edit]The 2-ketopentoses have two chiral centers; therefore, four (22) different stereoisomers are possible. The 3-ketopentoses are rare.
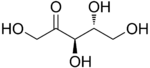 D-Ribulose |
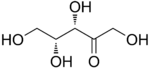 D-Xylulose |
 L-Ribulose |
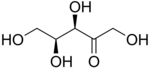 L-Xylulose |
Cyclic form
[edit]The closed or cyclic form of a pentose is created when thecarbonyl groupinteracts with ahydroxylin another carbon, turning the carbonyl into a hydroxyl and creating anether bridge–O– between the two carbons. Thisintramolecular reactionyields acyclicmolecule, with a ring consisting of one oxygen atom and usually four carbon atoms; the cyclic compounds are then calledfuranoses,for having the same rings as thecyclic ethertetrahydrofuran.[3]
The closure turns the carboxyl carbon into achiral center,which may have any of two configurations, depending on the position of the new hydroxyl. Therefore, each linear form can produce two distinct closed forms, identified by prefixes "α" and "β".
Deoxypentoses
[edit]The one deoxypentose has two total stereoisomers.
 D-Deoxyribose |
 L-Deoxyribose |
Properties
[edit]In the cell, pentoses have a highermetabolicstability thanhexoses.
Apolymercomposed of pentose sugars is called apentosan.
Tests for pentoses
[edit]The most important tests for pentoses rely on converting the pentose to furfural, which then reacts with achromophore.InTollens’ test for pentoses (not to be confused withTollens' silver-mirror testforreducing sugars), thefurfuralring reacts withphloroglucinolto produce a colored compound;[4]in theaniline acetate testwith aniline acetate;[5]and inBial's test,withorcinol.[6]In each of these tests, pentoses react much more strongly and quickly than hexoses.
References
[edit]- ^Pentose,Merriam-Webster
- ^"D-Ribose".PubChem compound webpage, accessed on 2010-02-06.
- ^abMorrison, Robert Thornton; Boyd, Robert Neilson.Organic Chemistry(2nd ed.). Allyn and Bacon.Library of Congress catalog 66-25695
- ^Oshima, Kintaro; Tollens, B. (May 1901)."Ueber Spectral‐Reactionen des Methylfurfurols".Berichte der Deutschen Chemischen Gesellschaft.34(2): 1425–1426.doi:10.1002/cber.19010340212.ISSN0365-9496.
- ^Seager, Spencer L.; Slabaugh, Michael R.; Hansen, Maren S. (2016-12-05).Safety Scale Laboratory Experiments.Cengage Learning. p. 358.ISBN9781337517140.
- ^Pavia, Donald L. (2005).Introduction to Organic Laboratory Techniques: A Small Scale Approach.Cengage Learning. p. 447.ISBN0534408338.
