Pi bond

Inchemistry,pi bonds(π bonds) arecovalentchemicalbonds,in each of which two lobes of anorbitalon oneatomoverlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has anelectron densityof zero at a sharednodal planethat passes through the two bondednuclei.This plane also is a nodal plane for themolecular orbitalof the pi bond. Pi bonds can form indoubleandtriple bondsbut do not form insingle bondsin most cases.
The Greek letterπin their name refers top orbitals,since theorbital symmetryof the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, thoughd orbitalsalso engage in pi bonding. This latter mode forms part of the basis formetal-metal multiple bonding.
Properties
[edit]
Pi bonds are usually weaker thansigma bonds.TheC-Cdouble bond, composed of one sigma and one pi bond,[1]has abond energyless than twice that of a C-C single bond, indicating that the stability added by the pi bond is less than the stability of a sigma bond. From the perspective ofquantum mechanics,this bond's weakness is explained by significantly less overlap between the component p-orbitals due to their parallel orientation. This is contrasted by sigma bonds which form bonding orbitals directly between the nuclei of the bonding atoms, resulting in greater overlap and a strong sigma bond.
Pi bonds result from overlap of atomic orbitals that are in contact through two areas of overlap. Most orbital overlaps that do not include the s-orbital, or have different internuclear axes (for example px+ pyoverlap, which does not apply to an s-orbital) are generally all pi bonds. Pi bonds are more diffuse bonds than the sigma bonds.Electronsin pi bonds are sometimes referred to aspi electrons.Molecular fragments joined by a pi bond cannot rotate about that bond without breaking the pi bond, because rotation involves destroying the parallel orientation of the constituent p orbitals.
Forhomonucleardiatomic molecules,bonding π molecular orbitals have only the one nodal plane passing through the bonded atoms, and no nodal planes between the bonded atoms. The correspondingantibonding,or π* ( "pi-star" ) molecular orbital, is defined by the presence of an additional nodal plane between these two bonded atoms.
Multiple bonds
[edit]A typicaldouble bondconsists of one sigma bond and one pi bond; for example, the C=C double bond inethylene(H2C=CH2). A typicaltriple bond,for example inacetylene(HC≡CH), consists of one sigma bond and two pi bonds in two mutually perpendicular planes containing the bond axis. Two pi bonds are the maximum that can exist between a given pair of atoms.Quadruple bondsare extremely rare and can be formed only betweentransition metalatoms, and consist of one sigma bond, two pi bonds and onedelta bond.
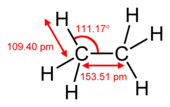
A pi bond is weaker than a sigma bond, but the combination of pi and sigma bond is stronger than either bond by itself. The enhanced strength of a multiple bond versus a single (sigma bond) is indicated in many ways, but most obviously by a contraction in bond lengths. For example, in organic chemistry, carbon–carbonbond lengthsare about 154pminethane,[2][3]134 pm in ethylene and 120 pm in acetylene. More bonds make the total bond length shorter and the bond becomes stronger.
|

|

|
| ethane(1 σ bond) | ethylene(1 σ bond + 1 π bond) | acetylene(1 σ bond + 2 π bonds) |
Special cases
[edit]A pi bond can exist between two atoms that do not have a net sigma-bonding effect between them.
In certainmetal complexes,pi interactions between a metal atom andalkyneandalkenepi antibonding orbitals form pi-bonds.
In some cases of multiple bonds between two atoms, there is no net sigma-bonding at all, only pi bonds. Examples include diiron hexacarbonyl (Fe2(CO)6),dicarbon(C2), anddiborane(2)(B2H2). In these compounds the central bond consists only of pi bonding because of a sigmaantibondaccompanying the sigma bond itself. These compounds have been used as computational models for analysis of pi bonding itself, revealing that in order to achieve maximumorbital overlapthe bond distances are much shorter than expected.[4]
See also
[edit]References
[edit]- ^Streitwieser, Andrew; Heathcock, Clayton H.; Kosower, Edward M. (1992).Introduction to organic chemistry.Heathcock, Clayton H., Kosower, Edward M. (4th ed.). New York: Macmillan. pp.250.ISBN978-0024181701.OCLC24501305.
- ^Veillard, A. (1970). "Relaxation during internal rotation ethane and hydrogen peroxyde".Theoretica Chimica Acta.18(1): 21–33.doi:10.1007/BF00533694.S2CID94310101.
- ^Harmony, Marlin D. (1990). "The equilibrium carbon–carbon single‐bond length in ethane".J. Chem. Phys.93(10): 7522–7523.Bibcode:1990JChPh..93.7522H.doi:10.1063/1.459380.
- ^Jemmis, E. D.;Pathak, Biswarup;King, R. Bruce;Schaefer III, Henry F.(2006). "Bond length and bond multiplicity: σ-bond prevents short π-bonds".Chemical Communications(20): 2164–2166.doi:10.1039/b602116f.PMID16703142.
