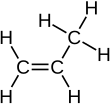
Propylene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1696878 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.693 | ||
| EC Number |
| ||
| 852 | |||
| KEGG | |||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1077 InLiquefied petroleum gas:1075 | ||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.081g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 1.81 kg/m3,gas (1.013 bar, 15 °C) 1.745 kg/m3,gas (1.013 bar, 25 °C) 613.9 kg/m3,liquid | ||
| Melting point | −185.2 °C (−301.4 °F; 88.0 K) | ||
| Boiling point | −47.6 °C (−53.7 °F; 225.6 K) | ||
| 0.61 g/m3 | |||
| -31.5·10−6cm3/mol | |||
| Viscosity | 8.34µPa·sat 16.7 °C | ||
| Structure | |||
| 0.366D(gas) | |||
| Hazards | |||
| GHSlabelling:[3] | |||
 
| |||
| Danger | |||
| H220 | |||
| P210,P377,P381,P403 | |||
| NFPA 704(fire diamond) | |||
| Flash point | −108 °C (−162 °F; 165 K) | ||
| Safety data sheet(SDS) | External MSDS | ||
| Related compounds | |||
Relatedalkenes;
related groups |
Ethylene,Isomers of Butylene; Allyl,Propenyl | ||
Related compounds
|
Propane,Propyne Propadiene,1-Propanol 2-Propanol | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Propylene,also known aspropene,is anunsaturatedorganic compoundwith thechemical formulaCH3CH=CH2.It has onedouble bond,and is the second simplest member of thealkeneclass ofhydrocarbons.It is a colorless gas with a faint petroleum-like odor.[4]
Propylene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust.[5]It was discovered in 1850 byA. W. von Hoffman's student Captain (later Major General[6])John Williams Reynoldsas the only gaseous product ofthermal decompositionofamyl alcoholto react with chlorine and bromine.[7]
Production
[edit]Steam cracking
[edit]The dominant technology for producing propylene issteam cracking,usingpropaneas thefeedstock.Cracking propane yields a mixture ofethylene,propylene,methane,hydrogen gas,and other related compounds. The yield of propylene is about 15%. The other principal feedstock is naphtha, especially in theMiddle Eastand Asia.[8] Propylene can be separated byfractional distillationfrom the hydrocarbon mixtures obtained from cracking and other refining processes; refinery-grade propene is about 50 to 70%.[9]In the United States,shale gasis a major source of propane.
Olefin conversion technology
[edit]In the Phillips triolefin orolefin conversion technology,propylene is interconverted withethyleneand2-butenes.Rheniumandmolybdenumcatalysts are used:[10]
The technology is founded on anolefin metathesisreaction discovered atPhillips Petroleum Company.[11][12]Propylene yields of about 90 wt% are achieved.
Related is theMethanol-to-Olefins/Methanol-to-Propeneprocess. It convertssynthesis gas (syngas)tomethanol,and thenconverts the methanol to ethylene and/or propene.The process produces water as a by-product.Synthesis gasis produced from the reformation of natural gas or by the steam-induced reformation of petroleum products such as naphtha, or bygasification of coalor natural gas.
Fluid catalytic cracking
[edit]High severityfluid catalytic cracking(FCC) uses traditional FCC technology under severe conditions (higher catalyst-to-oil ratios, higher steam injection rates, higher temperatures, etc.) in order to maximize the amount of propene and other light products. A high severity FCC unit is usually fed with gas oils (paraffins) and residues, and produces about 20–25% (by mass) of propene on feedstock together with greater volumes of motor gasoline and distillate byproducts. These high temperature processes are expensive and have a high carbon footprint. For these reasons, alternative routes to propylene continue to attract attention.[13]
Other commercialized methods
[edit]On-purpose propylene production technologies were developed throughout the twentieth century. Of these, propane dehydrogenation technologies such as the CATOFIN and OLEFLEX processes have become common, although they still make up a minority of the market, with most of the olefin being sourced from the above mentioned cracking technologies. Platinum, chromia, and vanadium catalysts are common in propane dehydrogenation processes.
Market
[edit]Propene production has remained static at around 35 milliontonnes(Europe and North America only) from 2000 to 2008, but it has been increasing in East Asia, most notably Singapore and China.[14]Total world production of propene is currently about half that of ethylene.
Research
[edit]The use of engineeredenzymeshas been explored but has not been commercialized.[15]
There is ongoing research into the use of oxygen carrier catalysts for the oxidative dehydrogenation of propane. This poses several advantages, as this reaction mechanism can occur at lower temperatures than conventional dehydrogenation, and may not be equilibrium-limited because oxygen is used to combust the hydrogen by-product.[16]
Uses
[edit]Propene is the second most important starting product in thepetrochemical industryafterethylene.It is the raw material for a wide variety of products.Polypropylenemanufacturers consume nearly two thirds of global production.[17]Polypropylene end uses include films, fibers, containers, packaging, and caps and closures. Propene is also used for the production of important chemicals such aspropylene oxide,acrylonitrile,cumene,butyraldehyde,andacrylic acid.In the year 2013 about 85 million tonnes of propene were processed worldwide.[17]
Propene andbenzeneare converted toacetoneandphenolvia thecumene process.
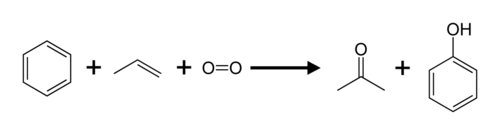
Propene is also used to produceisopropyl alcohol(propan-2-ol),acrylonitrile,propylene oxide,andepichlorohydrin.[18] The industrial production ofacrylic acidinvolves the catalytic partial oxidation of propene.[19]Propylene is an intermediate in the oxidation to acrylic acid.
In industry and workshops, propene is used as an alternative fuel to acetylene inOxy-fuel welding and cutting,brazing and heating of metal for the purpose of bending. It has become a standard inBernzOmaticproducts and others in MAPP substitutes,[20]now that trueMAPP gasis no longer available.
Reactions
[edit]Propene resembles other alkenes in that it undergoesadditionreactions relatively easily at room temperature. The relative weakness of its double bond explains its tendency to react with substances that can achieve this transformation. Alkene reactions include: 1)polymerization,2)oxidation,3)halogenationandhydrohalogenation,4)alkylation,5)hydration,6)oligomerization,and 7)hydroformylation.
Complexes of transition metals
[edit]Foundational to hydroformylation, alkene metathesis, and polymerization aremetal-propylene complexes,which are intermediates in these processes. Propylene isprochiral,meaning that binding of a reagent (such as a metal electrophile) to the C=C group yields one of twoenantiomers.
Polymerization
[edit]The majority of propene is used to form polypropylene, a very important commoditythermoplastic,throughchain-growth polymerization.[17]In the presence of a suitable catalyst (typically aZiegler–Natta catalyst), propene will polymerize. There are multiple ways to achieve this, such as using high pressures to suspending the catalyst in a solution of liquid propene, or running gaseous propene through afluidized bed reactor.[21]
Dimerization
[edit]In the presence ofcatalysts,propylenedimerizesto give2,3-dimethyl-1-buteneand/or2,3-dimethyl-2-butene.[22]
Environmental safety
[edit]Propene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust.[5]It is an impurity in some heating gases. Observed concentrations have been in the range of 0.1–4.8 parts per billion (ppb) in rural air, 4–10.5 ppb in urban air, and 7–260 ppb in industrial air samples.[9]
In the United States and some European countries athreshold limit valueof 500 parts per million (ppm) was established for occupational (8-hourtime-weighted average) exposure. It is considered avolatile organic compound(VOC) and emissions are regulated by many governments, but it is not listed by the U.S. Environmental Protection Agency (EPA) as ahazardous air pollutantunder theClean Air Act.With a relatively short half-life, it is not expected to bioaccumulate.[9]
Propene has low acute toxicity from inhalation and is not considered to be carcinogenic. Chronic toxicity studies in mice did not yield significant evidence suggesting adverse effects. Humans briefly exposed to 4,000 ppm did not experience any noticeable effects.[23]Propene is dangerous from its potential to displace oxygen as anasphyxiant gas,and from its high flammability/explosion risk.
Bio-propyleneis thebio-basedpropylene.[24][25] It has been examined, motivated by diverse interests such acarbon footprint.Production fromglucosehas been considered.[26]More advanced ways of addressing such issues focus on electrification alternatives tosteam cracking.
Storage and handling
[edit]Propene is flammable. Propene is usually stored as liquid under pressure, although it is also possible to store it safely as gas at ambient temperature in approved containers.[27]
Occurrence in nature
[edit]Propene is detected in theinterstellar mediumthrough microwave spectroscopy.[28]On September 30, 2013,NASAalso announced that the Cassini orbiter spacecraft, part of theCassini-Huygensmission, had discovered small amounts of naturally occurring propene in the atmosphere ofTitanusing spectroscopy.[29][30]
See also
[edit]References
[edit]- ^"General Principles, Rules, and Conventions".Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book).Cambridge:The Royal Society of Chemistry.2014. p. 31.doi:10.1039/9781849733069-00001.ISBN978-0-85404-182-4.
- ^Moss, G.P. (web version)."P-14.3 Locants".Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013.London: Queen Mary University. Section P-14.3.4.2 (d).Retrieved23 August2024.
- ^"Propylene".pubchem.ncbi.nlm.nih.gov.Retrieved14 December2021.
- ^"Propylene".
- ^abMorgott, David (2018-01-04)."The Human Exposure Potential from Propylene Releases to the Environment".International Journal of Environmental Research and Public Health.15(1): 66.doi:10.3390/ijerph15010066.ISSN1660-4601.PMC5800165.PMID29300328.
- ^"Maj Gen John Williams Reynolds, FCS".geni_family_tree.1816-12-25.Retrieved2023-12-30.
- ^Rasmussen, Seth C. (2018), Rasmussen, Seth C. (ed.),"Introduction",Acetylene and Its Polymers: 150+ Years of History,SpringerBriefs in Molecular Science, Cham: Springer International Publishing, pp. 1–19,doi:10.1007/978-3-319-95489-9_1,ISBN978-3-319-95489-9,retrieved2023-12-30
- ^Ashford's Dictionary of Industrial Chemicals, Third edition, 2011,ISBN978-0-9522674-3-0,pages 7766-9
- ^abc"Product Safety Assessment(PSA): Propylene".Dow Chemical Co. Archived fromthe originalon 2013-08-28.Retrieved2011-07-11.
- ^Ghashghaee, Mohammad (2018). "Heterogeneous catalysts for gas-phase conversion of ethylene to higher olefins".Rev. Chem. Eng.34(5): 595–655.doi:10.1515/revce-2017-0003.S2CID103664623.
- ^Banks, R. L.; Bailey, G. C. (1964). "Olefin Disproportionation. A New Catalytic Process".Industrial & Engineering Chemistry Product Research and Development.3(3): 170–173.doi:10.1021/i360011a002.
- ^Lionel Delaude; Alfred F. Noels (2005). "Metathesis".Kirk-Othmer Encyclopedia of Chemical Technology.Weinheim: Wiley-VCH.doi:10.1002/0471238961.metanoel.a01.ISBN978-0-471-23896-6.
- ^Schiffer, Zachary J.; Manthiram, Karthish (2017). "Electrification and Decarbonization of the Chemical Industry".Joule.1:10–14.doi:10.1016/j.joule.2017.07.008.hdl:1721.1/124019.S2CID117360588.
- ^Amghizar, Ismaël; Vandewalle, Laurien A.; Van Geem, Kevin M.; Marin, Guy B. (2017)."New Trends in Olefin Production".Engineering.3(2): 171–178.doi:10.1016/J.ENG.2017.02.006.
- ^de Guzman, Doris (October 12, 2012)."Global Bioenergies in bio-propylene".Green Chemicals Blog.
- ^Wu, Tianwei; Yu, Qingbo; Roghair; et al. (2020)."Chemical looping oxidative dehydrogenation of propane: A comparative study of Ga-based, Mo-based, V-based oxygen carriers".Chemical Engineering and Processing - Process Intensification.157:108137.Bibcode:2020CEPPI.15708137W.doi:10.1016/j.cep.2020.108137.ISSN0255-2701.
- ^abc"Market Study: Propylene (2nd edition), Ceresana, December 2014".ceresana.com.Retrieved2015-02-03.
- ^Budavari, Susan, ed. (1996). "8034. Propylene".The Merck Index, Twelfth Edition.New Jersey: Merck & Co. pp. 1348–1349.
- ^J.G.L., Fierro (Ed.) (2006).Metal Oxides, Chemistry and Applications.CRC Press. pp. 414–455.
- ^For example, "MAPP-Pro"
- ^Heggs, T. Geoffrey (2011-10-15),"Polypropylene",in Wiley-VCH Verlag GmbH & Co. KGaA (ed.),Ullmann's Encyclopedia of Industrial Chemistry,Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. o21_o04,doi:10.1002/14356007.o21_o04,ISBN978-3-527-30673-2,retrieved2021-07-09
- ^Olivier-Bourbigou, H.; Breuil, P. A. R.; Magna, L.; Michel, T.; Espada Pastor, M. Fernandez; Delcroix, D. (2020)."Nickel Catalyzed Olefin Oligomerization and Dimerization"(PDF).Chemical Reviews.120(15): 7919–7983.doi:10.1021/acs.chemrev.0c00076.PMID32786672.S2CID221124789.
- ^PubChem."Hazardous Substances Data Bank (HSDB): 175".pubchem.ncbi.nlm.nih.gov.Retrieved2021-07-09.
- ^Bio-based drop-in, smart drop-in and dedicated chemicals
- ^Duurzame bioplastics op basis van hernieuwbare grondstoffen
- ^Guzman, Doris de (12 October 2012)."Global Bioenergies in bio-propylene".Green Chemicals Blog.Retrieved2021-07-09.
- ^Encyclopedia of Chemical Technology, Fourth edition, 1996,ISBN0471-52689-4(v.20), page 261
- ^Marcelino, N.; Cernicharo, J.; Agúndez, M.; et al. (2007-08-10)."Discovery of Interstellar Propylene (CH2CHCH3): Missing Links in Interstellar Gas-Phase Chemistry".The Astrophysical Journal.665(2). IOP: L127–L130.arXiv:0707.1308.Bibcode:2007ApJ...665L.127M.doi:10.1086/521398.S2CID15832967.
- ^"Spacecraft finds propylene on Saturn moon, Titan".UPI.com. 2013-09-30.Retrieved2013-11-12.
- ^"Cassini finds ingredient of household plastic on Saturn moon".Spacedaily.com.Retrieved2013-11-12.




![{\displaystyle {\ce {CH2=CH2{}+CH3CH=CHCH3->[][{\text{Re, Mo}} \atop {\text{catalyst}}]2CH2=CHCH3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/acdd4b00da58144bb78872e0663521fe689b07b1)

