Protein 4.1
Protein 4.1,(Erythrocyte membrane protein band 4.1), is aproteinassociated with thecytoskeletonthat in humans is encoded by theEPB41gene.Protein 4.1 is a major structural element of theerythrocytemembraneskeleton. It plays a key role in regulating membrane physical properties of mechanical stability and deformability by stabilizingspectrin-actininteraction. Protein 4.1 (80 kD) interacts with spectrin and shortactin filamentsto form the erythrocyte membrane skeleton. Mutations of spectrin and protein 4.1 are associated withelliptocytosisorspherocytosisandanemiaof varying severity.
Clinical significance
[edit]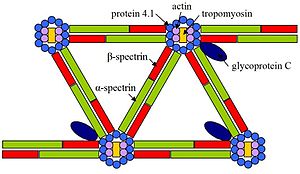
Elliptocytosis is a hematologic disorder characterized by elliptically shaped erythrocytes and a variable degree of hemolytic anemia. Inherited as an autosomal dominant, elliptocytosis results from mutation in any one of several genes encoding proteins of the red cell membrane skeleton. The form discussed here is the one found in the 1950s to be linked to Rh blood group and more recently shown to be caused by a defect in protein 4.1. 'Rh-unlinked' forms of elliptocytosis are caused by mutation in the alpha-spectringene (MIM 182860), the beta-spectringene (MIM 182870), or theband 3gene (MIM 109270) [supplied by OMIM].[5]
Interactions
[edit]Protein 4.1 has been shown tointeractwith:
See also
[edit]References
[edit]- ^abcGRCh38: Ensembl release 89: ENSG00000159023–Ensembl,May 2017
- ^abcGRCm38: Ensembl release 89: ENSMUSG00000028906–Ensembl,May 2017
- ^"Human PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Mouse PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Entrez Gene: EPB41 erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked)".
- ^Hung LY, Tang CJ, Tang TK (October 2000)."Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex".Mol. Cell. Biol.20(20): 7813–25.doi:10.1128/mcb.20.20.7813-7825.2000.PMC86375.PMID11003675.
- ^Hou CL, Tang Cj, Roffler SR, Tang TK (July 2000). "Protein 4.1R binding to eIF3-p44 suggests an interaction between the cytoskeletal network and the translation apparatus".Blood.96(2): 747–53.doi:10.1182/blood.V96.2.747.014k19_747_753(inactive 2024-04-10).PMID10887144.
{{cite journal}}:CS1 maint: DOI inactive as of April 2024 (link) - ^Mattagajasingh SN, Huang SC, Hartenstein JS, Snyder M, Marchesi VT, Benz EJ (April 1999)."A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein".J. Cell Biol.145(1): 29–43.doi:10.1083/jcb.145.1.29.PMC2148212.PMID10189366.
- ^Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ (September 2000)."Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton".J. Biol. Chem.275(39): 30573–85.doi:10.1074/jbc.M004578200.PMID10874042.
Further reading
[edit]- Conboy JG (1993). "Structure, function, and molecular genetics of erythroid membrane skeletal protein 4.1 in normal and abnormal red blood cells".Semin. Hematol.30(1): 58–73.PMID8434260.
- Calinisan V, Gravem D, Chen RP, Brittin S, Mohandas N, Lecomte MC, Gascard P (2006)."New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium".Front. Biosci.11:1646–66.doi:10.2741/1911.PMID16368544.S2CID26325962.
- Dalla Venezia N, Gilsanz F, Alloisio N, Ducluzeau MT, Benz EJ, Delaunay J (1992)."Homozygous 4.1(-) hereditary elliptocytosis associated with a point mutation in the downstream initiation codon of protein 4.1 gene".J. Clin. Invest.90(5): 1713–7.doi:10.1172/JCI116044.PMC443228.PMID1430200.
- Jöns T, Drenckhahn D (1992)."Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger".EMBO J.11(8): 2863–7.doi:10.1002/j.1460-2075.1992.tb05354.x.PMC556766.PMID1639060.
- Subrahmanyam G, Bertics PJ, Anderson RA (1991)."Phosphorylation of protein 4.1 on tyrosine-418 modulates its function in vitro".Proc. Natl. Acad. Sci. U.S.A.88(12): 5222–6.Bibcode:1991PNAS...88.5222S.doi:10.1073/pnas.88.12.5222.PMC51844.PMID1647028.
- Conboy JG, Chan JY, Chasis JA, Kan YW, Mohandas N (1991)."Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1".J. Biol. Chem.266(13): 8273–80.doi:10.1016/S0021-9258(18)92973-X.PMID2022644.
- Horne WC, Prinz WC, Tang EK (1990). "Identification of two cAMP-dependent phosphorylation sites on erythrocyte protein 4.1".Biochim. Biophys. Acta.1055(1): 87–92.doi:10.1016/0167-4889(90)90095-U.PMID2171679.
- Conboy J, Marchesi S, Kim R, Agre P, Kan YW, Mohandas N (1990)."Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. II. Determination of molecular genetic origins of rearrangements".J. Clin. Invest.86(2): 524–30.doi:10.1172/JCI114739.PMC296755.PMID2384598.
- Inaba M, Maede Y (1989)."O-N-acetyl-D-glucosamine moiety on discrete peptide of multiple protein 4.1 isoforms regulated by alternative pathways".J. Biol. Chem.264(30): 18149–55.doi:10.1016/S0021-9258(19)84689-6.PMID2808371.
- Korsgren C, Cohen CM (1988)."Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3".J. Biol. Chem.263(21): 10212–8.doi:10.1016/S0021-9258(19)81500-4.PMID2968981.
- Conboy JG, Chan J, Mohandas N, Kan YW (1988)."Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells".Proc. Natl. Acad. Sci. U.S.A.85(23): 9062–5.Bibcode:1988PNAS...85.9062C.doi:10.1073/pnas.85.23.9062.PMC282663.PMID3194408.
- Tang TK, Leto TL, Marchesi VT, Benz EJ (1988). "Expression of Specific Isoforms of Protein 4.1 in Erythroid and Non-Erythroid Tissues".Molecular Biology of Hemopoiesis.Advances in Experimental Medicine and Biology. Vol. 241. pp. 81–95.doi:10.1007/978-1-4684-5571-7_12.ISBN978-1-4684-5573-1.PMID3223413.
- Tang TK, Leto TL, Correas I, Alonso MA, Marchesi VT, Benz EJ (1988)."Selective expression of an erythroid-specific isoform of protein 4.1".Proc. Natl. Acad. Sci. U.S.A.85(11): 3713–7.Bibcode:1988PNAS...85.3713T.doi:10.1073/pnas.85.11.3713.PMC280288.PMID3375238.
- Conboy J, Kan YW, Shohet SB, Mohandas N (1987)."Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton".Proc. Natl. Acad. Sci. U.S.A.83(24): 9512–6.doi:10.1073/pnas.83.24.9512.PMC387170.PMID3467321.
- Correas I, Speicher DW, Marchesi VT (1986)."Structure of the spectrin-actin binding site of erythrocyte protein 4.1".J. Biol. Chem.261(28): 13362–6.doi:10.1016/S0021-9258(18)69313-5.PMID3531202.
- Tchernia G, Mohandas N, Shohet SB (1981)."Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability".J. Clin. Invest.68(2): 454–60.doi:10.1172/JCI110275.PMC370818.PMID6894932.
- Schischmanoff PO, Winardi R, Discher DE, Parra MK, Bicknese SE, Witkowska HE, Conboy JG, Mohandas N (1995)."Defining of the minimal domain of protein 4.1 involved in spectrin-actin binding".J. Biol. Chem.270(36): 21243–50.doi:10.1074/jbc.270.36.21243.PMID7673158.
- Lue RA, Marfatia SM, Branton D, Chishti AH (1994)."Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1".Proc. Natl. Acad. Sci. U.S.A.91(21): 9818–22.Bibcode:1994PNAS...91.9818L.doi:10.1073/pnas.91.21.9818.PMC44908.PMID7937897.
- Conboy JG, Chasis JA, Winardi R, Tchernia G, Kan YW, Mohandas N (1993)."An isoform-specific mutation in the protein 4.1 gene results in hereditary elliptocytosis and complete deficiency of protein 4.1 in erythrocytes but not in nonerythroid cells".J. Clin. Invest.91(1): 77–82.doi:10.1172/JCI116203.PMC329997.PMID8423235.
External links
[edit]- erythrocyte+membrane+band+4.1+proteinat the U.S. National Library of MedicineMedical Subject Headings(MeSH)









