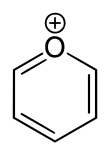
Pyrylium
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyrylium[1] | |||
| Other names
Pyranium
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1421881 | |||
| ChEBI |
| ||
| ChemSpider | |||
| 558560 | |||
PubChemCID
|
|||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5O+ | |||
| Molar mass | 81.09 g/mol | ||
| Related compounds | |||
Related compounds
|
thiopyrylium,selenopyrylium,telluropyrylium | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Pyryliumis acation(positiveion) with formulaC5H5O+,consisting of a six-membered ring of fivecarbonatoms, each with onehydrogenatom, and one positively chargedoxygenatom. The bonds in the ring areconjugatedas inbenzene,giving it anaromaticcharacter. In particular, because of the positive charge, the oxygen atom istrivalent.Pyrilium is a mono-cyclicandheterocycliccompound, one of theoxonium ions.
Synthesis
[edit]Pyrylium salts are easily produced from simple starting materials through acondensation reaction.[2]
Pyrylium salts with aromatic substituents, such 2,4,6-triphenylpyrylium tetrafluoroborate, can be obtained from two moles ofacetophenone,one mole ofbenzaldehyde,and excesstetrafluoroboric acid.[3]For pyrylium salts with alkyl substituents, such as 2,4,6-trimethylpyrylium salts, the best method uses theBalaban-Nenitzescu-Praill synthesis fromtertiary butanolandacetic anhydridein the presence of tetrafluoroboric,[4]perchloric,[5]or trifluoromethanesulfonic acids.[6]
Hydroxide bases open and hydrolyzepyridineto an enedione base that cyclizes in very strong acids to a pyrylium cation.[7]
Enolizing conditions (strong acid) force pyrones to their pyrylium tautomer.[8]
Chemical properties
[edit]Pyrylium and its derivatives form stablesaltswith a variety of anions.[9][10][11][12][13][14]
Like otheroxonium ions,pyrylium is unstable in neutral water. However, pyrylium is much less reactive than ordinary oxonium ions because of aromatic stabilization. The highly electronegative oxygen strongly perturbs the orbitals in the aromatic ring, and pyrylium derivatives are extremely resistant toelectrophilic aromatic substitution.Pyrylium cations react withnucleophilesat theorthoandparapositions,typically throughANRORC.[15]
2,4,6-Triphenylpyrylium salts are converted by hydroxide bases into a stable 1,5-enedione (pseudobase), but 2,4,6-trimethylpyrylium salts on treatment with hot alkali hydroxides afford an unstable pseudobase that undergoes an intramolecular condensation yielding 3,5-dimethylphenol.In warm deuterium oxide, 2,4,6-trimethylpyrylium salts undergo isotopic exchange of 4-methyl hydrogens faster than for the 2- and 6-methyl groups, allowing the synthesis of regioselectively deuterated compounds.[citation needed]
Derivatives
[edit]Pyrylium's electrophilicity makes them useful materials for producing other compounds with stronger aromatic character. Pyrylium salts affordpyridineswithammonia,[16]pyridiniumsalts with primary amines,pyridine-N-oxideswithhydroxylamine,phosphabenzeneswithphosphinederivatives,thiopyryliumsalts withhydrogen sulfide,and benzene derivatives withacetonitrileornitromethane.
Many important cations are formally derived from pyrylium by substitution of variousfunctional groupsfor some or all the hydrogens in the ring.2,4,6-Triphenylpyryliumreacts withprimary aminesto givepyridiniumderivatives called "Katritzkysalts "; they are commonly used in metal-catalyzednucleophilic displacementof the amine.[15]
Pyrones
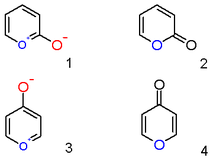
[edit]A pyrylium cation with ahydroxylanionsubstituentin the 2-position is not thezwitterionicaromatic compound (1), but the neutralunsaturatedlactone2-pyroneor pyran-2-one (2). Important representatives of this class are thecoumarins.Likewise a 4-hydroxyl pyrylium compound is aγ-pyroneor pyran-4-one (4), to which group belong compounds such asmaltol.
2-Pyrones are known to react withalkynesin aDiels–Alder reactionto formarenecompounds with expulsion ofcarbon dioxide,for example:[17]

Polycyclic oxonium arenes
[edit]Chromenylium ion
[edit]
Onebicyclicpyrylium ion is called benzopyrylium ion (IUPAC:chromenylium ion) (formula:C9H7O+,molar mass: 131.15 g/mol, exact mass: 131.04968983). It can be seen as a charged derivative of 2H-1-benzopyran(IUPAC: 2H-chromene,C9H8O), or a (charged) substituted heterocyclic derivative ofnaphthalene(C10H8).

In biology, the 2-phenylbenzopyrylium (2-phenylchromenylium) ion is referred to asflavylium.A class of flavylium-derived compounds areanthocyanidinsandanthocyanins,pigments that are responsible for the colors of many flowers.[citation needed]
Naphthoxanthenium cation
[edit]
Higherpolycyclicderivatives of pyrylium also exist. One good example isnaphthoxanthenium.This dye is highly stable, aromatic, and planar. It absorbs in the UV and blue region and presents exceptional photophysical properties. It can be synthesized by chemical or photochemical reactions.[18]
See also
[edit]- 6-membered aromatic rings with one carbon replaced by another group:borabenzene,silabenzene,germabenzene,stannabenzene,pyridine,phosphorine,arsabenzene,stibabenzene,bismabenzene,thiopyrylium,selenopyrylium,telluropyrylium
- Pyran,C5H6O(pyrones lacking the ketone group)
References
[edit]- ^International Union of Pure and Applied Chemistry(2014).Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013.The Royal Society of Chemistry.p. 1097.doi:10.1039/9781849733069.ISBN978-0-85404-182-4.
- ^Balaban, A. T.; Wray, V. (1977). "13C n.m.r. spectra of some pyrylium salts and related compounds ".Organic Magnetic Resonance.9(1): 16–22.doi:10.1002/mrc.1270090105.
- ^Dimroth, Karl; Reichardt, C.; Vogel, K. (1969)."2,4,6-Triphenylpyrylium tetrafluoroborate".Organic Syntheses.49;Collected Volumes,vol. 5.
- ^Balatan, A. T.; Boulton, A. J. (1969)."2,4,6-Trimethylpyrylium tetrafluoroborate".Organic Syntheses.49;Collected Volumes,vol. 5, pp. 1112–1113.
- ^Balaban, Alexandru T.; Nenitzescu, Costin D. (1968)."2,4,6-Trimethylpyrylium perchlorate".Organic Syntheses.44;Collected Volumes,vol. 5, pp. 1088, 1106, 1114, 1135.
- ^Balaban, Alexandru T.; Boulton, A. J. (1973)."2,4,6-Trimethylpyrylium trifluoromethanesulfonate".Organic Syntheses;Collected Volumes,vol. 5, pp. 1112, 1114–1116.
- ^Gómez-Palomino, Alejandro; Ghiazza, Clément; Busch, Julia; Wagner, Lucas; Cornella, Josep (2023)."Preparation of Pyrylium tetrafluoroborate (Pyry-BF4) ".Organic Syntheses.100:361–381.
- ^Agyemang, Nana B.; Murelli, Ryan P. (2019)."Synthesis of 5-hydroxy-4-methoxy-2-methylpyrylium trifluoromethanesulfonate from Kojic acid".Organic Syntheses.96:494–510.
- ^Gilchrist, T. L. (1997).Heterocyclic Chemistry.ISBN0-582-27843-0.
- ^Balaban, A. T.; Schroth, W.; Fischer, G. (1969). Katritzky, A. R.; Boulton, A. J. (eds.).Pyrylium Salts. I. Synthesis.Advances in Heterocyclic Chemistry. Vol. 10. New York: Academic Press. pp. 241–326.doi:10.1016/S0065-2725(08)60499-7.
- ^Balaban, A. T.; Dinculescu, A.; Dorofeenko, G. N.; Fischer, G. W.; Koblik, A. V.; Mezheritskii, V. V.; Schroth, W. (1982). Katritzky, A. R. (ed.).Pyrylium Salts. Syntheses, Reactions and Physical Properties.Advances in Heterocyclic Chemistry: Supplement. Vol. 2. New York: Academic Press.ISBN978-0-12-020652-0.
- ^Balaban, A. T. (1979)."The Pyrylium Cation as a Synthon in Organic Chemistry".In Mitra, R. B.; Ayyangar, N. R.; Gogte, V. N.; Acheson, R. M.; Cromwell, N. (eds.).New Trends in Heterocyclic Chemistry.Studies in Organic Chemistry. Vol. 3. Amsterdam: Elsevier. pp.79–111.ISBN978-0-444-41737-4.
- ^Balaban, A. T. (1987). "Pyrylium Salts as Useful Synthons". In Chizov, O. (ed.).Organic Synthesis: Modern Trends.Oxford: Blackwell. pp. 263–274.ISBN0-632-02014-8.
- ^Balaban, T. S.; Balaban, A. T. (2003). "Pyrylium Salts".Hetarenes and Related Ring Systems, Six-membered Hetarenes with one Chalcogen.Science of Synthesis; Houben-Weyl Methods of Molecular Transformations. Vol. 14. Stuttgart: Georg Thieme Verlag. pp. 11–200.ISBN978-3-13-118641-6.
- ^abPang, Yue; Moser, Daniel; Cornella, Josep (2020)."Pyrylium Salts: Selective Reagents for the Activation of Primary Amino Groups in Organic Synthesis".Synthesis.52(4): 489–503.doi:10.1055/s-0039-1690703.S2CID208705148.
- ^Anderson, A. G.; Stang, P. J. (1981)."2,6-Di-tert-Butyl-4-Methylpyridine "(PDF).Organic Syntheses.60:34;Collected Volumes,vol. 7, p. 144.
- ^Delaney, P. M.; Moore, J. E.; Harrity, J. P. A. (2006). "An Alkynylboronic Ester Cycloaddition Route to Functionalised Aromatic Boronic Esters".Chemical Communications.2006(31): 3323–3325.doi:10.1039/b607322k.PMID16883424.
- ^Bucher, G.; Bresolí-Obach, R.; Brosa, C.; Flors, C.; Luis, J. L.; Grillo, T. A.; Nonell, S. (2014). "β-Phenyl quenching of 9-phenylphenalenones: a novel photocyclisation reaction with biological implications".Physical Chemistry Chemical Physics.16(35): 18813–18820.Bibcode:2014PCCP...1618813B.doi:10.1039/C4CP02783C.PMID25079707.