Radical anion
Inorganic chemistry,aradical anionis afree radicalspecies[1]that carries anegative charge.Radicalanionsare encountered inorganic chemistryas reduced derivatives of polycyclic aromatic compounds, e.g.sodium naphthenide.An example of a non-carbon radical anion is thesuperoxideanion, formed by transfer of one electron to anoxygenmolecule. Radical anions are typically indicated by.
Polycyclic radical anions
[edit]Manyaromaticcompounds can undergoone-electron reductionbyalkali metals.The electron is transferred from the alkali metal ion to an unoccupied antibonding p-p п* orbital of the aromatic molecule. This transfer is usually only energetically favorable if the aprotic solvent efficiently solvates the alkali metal ion. Effective solvents are those that bind to the alkali metal cation:diethyl ether<THF<1,2-dimethoxyethane<HMPA.In principle any unsaturated molecule can form a radical anion, but the antibonding orbitals are only energetically accessible in more extensive conjugated systems. Ease of formation is in the orderbenzene<naphthalene<anthracene<pyrene,etc. Salts of the radical anions are often not isolated as solids but used in situ. They are usually deeply colored.
- Naphthalenein the form of
- Lithium naphthaleneis obtained from the reaction of naphthalene withlithium.
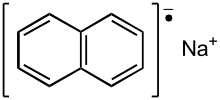
- Sodium naphthaleneis obtained from the reaction ofnaphthalenewithsodium.
- Sodium1-methylnaphthaleneand 1-methylnaphthalene are more soluble than sodium naphthalene and naphthalene, respectively.[2]
- biphenylas its lithium salt.[3]
- acenaphthyleneis a milder reductant than the naphthalene anion.
- anthracenein the form of its alkali metal salts.[4]
- pyreneas its sodium salt.[5]
- Perylenein the form of its alkali metal (M = Li, Na, Cs) etherates.[6]
Other examples
[edit]Cyclooctatetraeneis reduced by elementalpotassiumto the dianion. The resulting dianion is a 10-pi electron system, which conforms to theHuckel ruleforaromaticity.Quinoneis reduced to asemiquinoneradical anion.Semidionesare derived from the reduction of dicarbonyl compounds.
Reactions
[edit]Redox
[edit]The pi-radical anions are used as reducing agents in specialized syntheses. Being soluble in at least some solvents, these salts act faster than the alkali metals themselves. The disadvantages are that the polycyclic hydrocarbon must be removed. The reduction potential of alkali metal naphthalene salts is about 3.1 V (vs Fc+/0). The reduction potentials of the larger systems are lower, for example acenaphthalene is 2.45 V.[7]Many radical anions are susceptible to further reduction to dianions.
| hydrocarbon | M+ | E1/2 | comments |
|---|---|---|---|
| naphthalene | Li+ | -3.09 V | can be reduced to dianion |
| naphthalene | Na+ | -3.09 V | |
| biphenyl | Li+ | -3.18 V | |
| anthracene | Na+ | -2.53 V | |
| perylene | Na+ | -2.19 V | includes dme solvate |
Protonation
[edit]Addition of a proton source (even water) to a radical anion results in protonation, i.e. the sequence of reduction followed by protonation is equivalent tohydrogenation.For instance, the anthracene radical anion forms mainly (but not exclusively) 9,10-dihydroanthracene. Radical anions and their protonation are central to theBirch reduction.
Coordination to metal ions
[edit]Radical anions of polycyclic aromatic compounds function as ligands inorganometallic chemistry.[8]
Radical cations
[edit]Cationic radical species are much less common than the anions. Denoted,they appear prominently in mass spectrometry.[9]When a gas-phase molecule is subjected toelectron ionizationone electron is abstracted by an electron in the electron beam to create a radical cation M+..This species represents themolecular ionor parent ion. A typicalmass spectrumshows multiple signals because the molecular ion fragments into a complex mixture of ions and uncharged radical species. For example, themethanolradical cation fragments into ametheniumcationCH+3and ahydroxylradical. Innaphthalenethe unfragmented radical cation is by far the most prominent peak in the mass spectrum. Secondary species are generated fromprotongain (M+1) and proton loss (M-1).
Some compounds containing thedioxygenylcation can be prepared in bulk.[10]
Organic conductors
[edit]Radical cations figure prominently in the chemistry and properties ofconducting polymers.Such polymers are formed by the oxidation ofheterocyclesto give radical cations, which condense with the parent heterocycle. For example,polypyrroleis prepared by oxidation ofpyrroleusingferric chloridein methanol:
- nC4H4NH + 2 FeCl3→ (C4H2NH)n+ 2 FeCl2+ 2 HCl
Once formed, these polymers become conductive upon oxidation.[11]Polaronsandbipolaronsare radical cations encountered in doped conducting polymers.
References
[edit]- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "radical ion".doi:10.1351/goldbook.R05073
- ^Liu, X.; Ellis, J. E. (2004). "Hexacarbonylvanadate(1−) and Hexacarbonylvanadium(0)".Inorg. Synth.34:96–103.doi:10.1002/0471653683.ch3.ISBN0-471-64750-0.
- ^Rieke, Reuben D.; Wu, Tse-Chong & Rieke, Loretta I. (1995). "Highly Reactive Calcium for the Preparation of Organocalcium Reagents: 1-Adamantyl Calcium Halides and Their Addition to Ketones: 1-(1-Adamantyl)cyclohexanol".Org. Synth.72:147.doi:10.15227/orgsyn.072.0147.
- ^abCastillo, Maximiliano; Metta-Magaña, Alejandro J.; Fortier, Skye (2016). "Isolation of Gravimetrically Quantifiable Alkali Metal Arenides Using 18-Crown-6".New Journal of Chemistry.40(3): 1923–1926.doi:10.1039/C5NJ02841H.
- ^Kucera, Benjamin E.; Jilek, Robert E.; Brennessel, William W.; Ellis, John E. (2014). "Bis(pyrene)metal complexes of vanadium, niobium and titanium: Isolable homoleptic pyrene complexes of transition metals".Acta Crystallographica Section C: Structural Chemistry.70(8): 749–753.doi:10.1107/S2053229614015290.PMID25093352.
- ^Näther, Christian; Bock, Hans; Havlas, Zdenek; Hauck, Tim (1998). "Solvent-Shared and Solvent-Separated Ion Multiples of Perylene Radical Anions and Dianions: An Exemplary Case of Alkali Metal Cation Solvation".Organometallics.17(21): 4707–4715.doi:10.1021/om970610g.
- ^Connelly, Neil G.; Geiger, William E. (1996). "Chemical Redox Agents for Organometallic Chemistry".Chemical Reviews.96(2): 877–910.doi:10.1021/cr940053x.PMID11848774.
- ^Ellis, John E. (2019). "The Chatt Reaction: Conventional Routes to homoleptic Arenemetalates of d-Block Elements".Dalton Transactions.48(26): 9538–9563.doi:10.1039/C8DT05029E.PMID30724934.S2CID73436073.
- ^Sparkman, O. David (2000).Mass spectrometry desk reference.Pittsburgh: Global View Pub. p. 53.ISBN978-0-9660813-2-9.
- ^Solomon, I. J.; Brabets, R. I.; Uenishi, R. K.; Keith, J. N.; McDonough, J. M. (1964). "New Dioxygenyl Compounds".Inorganic Chemistry.3(3): 457.doi:10.1021/ic50013a036.
- ^"Polypyrrole: a conducting polymer; its synthesis, properties and applications" Russ. Chem. Rev. 1997, vol. 66, p.443ff.(http://iopscience.iop.org/0036-021X/66/5/R04)


