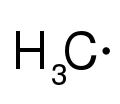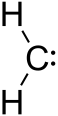Reactive intermediate
Inchemistry,areactive intermediateor anintermediateis a short-lived, high-energy, highly reactivemolecule.When generated in achemical reaction,it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures,matrix isolation.When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place.[1][2][3][4]
Most chemical reactions take more than oneelementary stepto complete, and a reactive intermediate is a high-energy, hence unstable, product that exists only in one of the intermediate steps. The series of steps together make areaction mechanism.A reactive intermediate differs from a reactant or product or a simplereaction intermediateonly in that it cannot usually be isolated but is sometimes observable only through fastspectroscopicmethods. It is stable in the sense that anelementary reactionforms the reactive intermediate and the elementary reaction in the next step is needed to destroy it.
When a reactive intermediate is not observable, its existence must beinferredthrough experimentation. This usually involves changing reaction conditions such as temperature or concentration and applying the techniques ofchemical kinetics,chemical thermodynamics,orspectroscopy.Reactive intermediates based on carbon areradicals,carbenes,carbocations,carbanions,arynes,andcarbynes.
Common features
[edit]Reactive intermediates have several features in common:
- lowconcentrationwith respect to reaction substrate and final reaction product
- with the exception of carbanions, these intermediates do not obey thelewis octet rule,hence the high reactivity
- often generated onchemical decompositionof achemical compound
- it is often possible to prove the existence of this species byspectroscopicmeans
- cage effectshave to be taken into account
- often stabilisation byconjugationorresonance
- often difficult to distinguish from atransition state
- prove existence by means ofchemical trapping
Carbon
[edit]-
Radical
-
Carbene
-
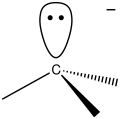
Carbocation
-
Carbanion
-
Carbyne
-
Benzyne (an aryne)
Other reactive intermediates
[edit]- Carbenoid
- Ion-neutral complex
- Keto anions
- Nitrenes
- Oxocarbeniumions
- Phosphinidenes
- Phosphoryl nitride
- Tetrahedral intermediatesincarbonyladdition reactions
See also
[edit]References
[edit]- ^Carey, Francis A.; Sundberg, Richard J.; (1984). Advanced Organic Chemistry Part A Structure and Mechanisms (2nd ed.). New York N.Y.: Plenum Press.ISBN0-306-41198-9.
- ^March Jerry; (1992). Advanced Organic Chemistry reactions, mechanisms and structure (4th ed.). New York: John Wiley & SonsISBN0-471-60180-2
- ^Gilchrist, T. L. (1966).Carbenes nitrenes and arynes.Springer US.ISBN9780306500268.
- ^Moss, Robert A.; Platz, Matthew S.; Jones, Jr., Maitland (2004).Reactive intermediate chemistry.Hoboken, N.J.: Wiley-Interscience.ISBN9780471721499.
Extranol links
[edit] Media related toReactive intermediatesat Wikimedia Commons
Media related toReactive intermediatesat Wikimedia Commons