Redox
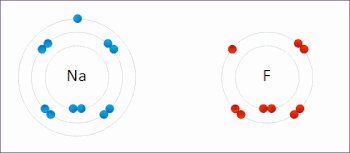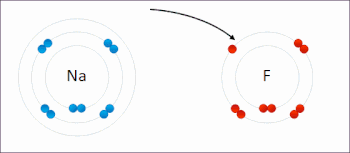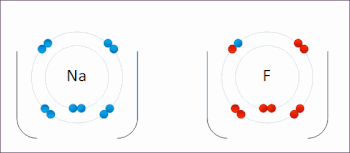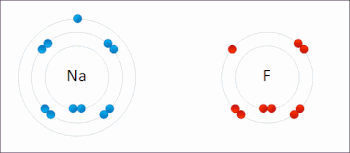
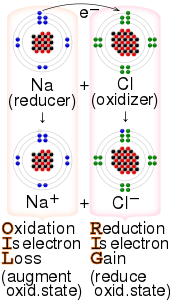
Redox(/ˈrɛdɒks/RED-oks,/ˈriːdɒks/REE-doks,reduction–oxidation[2]oroxidation–reduction[3]: 150 ) is a type ofchemical reactionin which theoxidation statesof thereactantschange.[4]Oxidation is the loss ofelectronsor an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction.
There are two classes of redox reactions:
- Electron-transfer– Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials.
- Atom transfer – An atom transfers from onesubstrateto another. For example, in therustingofiron,the oxidation state of iron atoms increases as the iron converts to anoxide,and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with forming oxides, other chemical species can serve the same function.[5]Inhydrogenation,bonds likeC=Care reduced bytransfer of hydrogen atoms.
Terminology
[edit]"Redox" is aportmanteauof the words "reduction" and "oxidation." The term "redox" was first used in 1928.[6]
Oxidation is a process in which a substance loses electrons. Reduction is a process in which a substance gains electrons.
The processes of oxidation and reduction occur simultaneously and cannot occur independently.[5]In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant orreducing agentloses electrons and is oxidized, and the oxidant oroxidizing agentgains electrons and is reduced. The pair of an oxidizing and reducing agent that is involved in a particular reaction is called a redox pair. A redox couple is a reducing species and its corresponding oxidizing form,[7]e.g.,Fe2+
/Fe3+
.The oxidation alone and the reduction alone are each called ahalf-reactionbecause two half-reactions always occur together to form a whole reaction.[5]
Oxidants
[edit]Oxidation originally implied a reaction with oxygen to form an oxide. Later, the term was expanded to encompasssubstancesthat accomplished chemical reactions similar to those of oxygen. Ultimately, the meaning was generalized to include all processes involving the loss of electrons or the increase in the oxidation state of a chemical species.[8]: A49 Substances that have the ability to oxidize other substances (cause them to lose electrons) are said to be oxidative or oxidizing, and are known asoxidizing agents,oxidants, or oxidizers. The oxidant removes electrons from another substance, and is thus itself reduced.[8]: A50 Because it "accepts" electrons, the oxidizing agent is also called anelectron acceptor.Oxidants are usually chemical substances with elements in high oxidation states[3]: 159 (e.g.,N
2O
4,MnO−
4,CrO
3,Cr
2O2−
7,OsO
4), or else highlyelectronegativeelements (e.g.O2,F2,Cl2,Br2,I2) that can gain extra electrons by oxidizing another substance.[3]: 909
Oxidizers are oxidants, but the term is mainly reserved for sources of oxygen, particularly in the context of explosions.Nitric acidis a strong oxidizer.[9]

Reductants
[edit]Substances that have the ability to reduce other substances (cause them to gain electrons) are said to be reductive or reducing and are known asreducing agents,reductants, or reducers. The reductant transfers electrons to another substance and is thus itself oxidized.[3]: 159 Because it donates electrons, the reducing agent is also called anelectron donor.Electron donors can also formcharge transfer complexeswith electron acceptors. The word reduction originally referred to the loss in weight upon heating a metallicoresuch as ametal oxideto extract the metal. In other words, ore was "reduced" to metal.[10]Antoine Lavoisierdemonstrated that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of reduction then became generalized to include all processes involving a gain of electrons.[10]Reducing equivalent refers tochemical specieswhich transfer the equivalent of oneelectronin redox reactions. The term is common inbiochemistry.[11]A reducing equivalent can be an electron or a hydrogen atom as ahydride ion.[12]
Reductants in chemistry are very diverse.Electropositiveelementalmetals,such aslithium,sodium,magnesium,iron,zinc,andaluminium,are good reducing agents. These metals donate electrons relatively readily.[13]
Hydride transfer reagents,such asNaBH4andLiAlH4,reduce by atom transfer: they transfer the equivalent of hydride or H−.These reagents are widely used in the reduction ofcarbonylcompounds toalcohols.[14][15]A related method of reduction involves the use of hydrogen gas (H2) as sources of H atoms.[3]: 288
Electronation and deelectronation
[edit]TheelectrochemistJohn Bockrisproposed the words electronation and de-electronation to describe reduction and oxidation processes, respectively, when they occur atelectrodes.[16]These words are analogous toprotonationanddeprotonation.[17]They have not been widely adopted by chemists worldwide,[citation needed]althoughIUPAChas recognized the terms electronation[18]and de-electronation.[19]
Rates, mechanisms, and energies
[edit]This sectionneeds expansion.You can help byadding to it.(April 2023) |
Redox reactions can occur slowly, as in the formation ofrust,or rapidly, as in the case of burningfuel.Electron transfer reactions are generally fast, occurring within the time of mixing.[20]
The mechanisms of atom-transfer reactions are highly variable because many kinds of atoms can be transferred. Such reactions can also be quite complex, involving many steps. The mechanisms of electron-transfer reactions occur by two distinct pathways,inner sphere electron transfer[21]andouter sphere electron transfer.[22]
Analysis of bond energies andionization energiesin water allows calculation of the thermodynamic aspects of redox reactions.[23]
Standard electrode potentials (reduction potentials)
[edit]This sectionneeds additional citations forverification.(December 2023) |
Each half-reaction has a standardelectrode potential(Eo
cell), which is equal to the potential difference orvoltageat equilibrium understandard conditionsof anelectrochemical cellin which thecathodereaction is thehalf-reactionconsidered, and theanodeis astandard hydrogen electrodewhere hydrogen is oxidized:
- 1⁄2H2→ H++ e−
The electrode potential of each half-reaction is also known as its reduction potential (Eo
red), or potential when the half-reaction takes place at a cathode. The reduction potential is a measure of the tendency of the oxidizing agent to be reduced. Its value is zero for H++ e−→1⁄2H2by definition, positive for oxidizing agents stronger than H+(e.g., +2.866 V for F2) and negative for oxidizing agents that are weaker than H+(e.g., −0.763V for Zn2+).[8]: 873
For a redox reaction that takes place in a cell, the potential difference is:
- Eo
cell=Eo
cathode–Eo
anode
However, the potential of the reaction at the anode is sometimes expressed as anoxidation potential:
- Eo
ox= –Eo
red
The oxidation potential is a measure of the tendency of the reducing agent to be oxidized but does not represent the physical potential at an electrode. With this notation, the cell voltage equation is written with a plus sign
- Eo
cell=Eo
red(cathode)+Eo
ox(anode)
Examples of redox reactions
[edit]
In the reaction betweenhydrogenandfluorine,hydrogen is being oxidized and fluorine is being reduced:
- H2+ F2→ 2 HF
This spontaneous reaction releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the F-F bond. This reaction can be analyzed as twohalf-reactions.The oxidation reaction converts hydrogen toprotons:
The reduction reaction convertsfluorineto the fluoride anion:
- F2+ 2 e−→ 2F−
The half-reactions are combined so that the electrons cancel:
H
2→ 2 H++ 2 e− F
2+ 2 e−→ 2 F−
H2+ F2 → 2 H++ 2 F−
The protons and fluoride combine to formhydrogen fluoridein a non-redox reaction:
- 2 H++ 2 F−→ 2 HF
The overall reaction is:
- H2+ F2→ 2 HF
Metal displacement
[edit]
In this type of reaction, ametalatom in a compound or solution is replaced by an atom of another metal. For example,copperis deposited whenzincmetal is placed in acopper(II) sulfatesolution:
- Zn (s) + CuSO4(aq) → ZnSO4(aq) + Cu (s)
In the above reaction, zinc metal displaces the copper(II) ion from the copper sulfate solution, thus liberating free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc.
The ionic equation for this reaction is:
- Zn + Cu2+→ Zn2++ Cu
As twohalf-reactions,it is seen that the zinc is oxidized:
- Zn → Zn2++ 2 e−
And the copper is reduced:
- Cu2++ 2 e−→ Cu
Other examples
[edit]- The reduction ofnitratetonitrogenin the presence of an acid (denitrification):
- 2 NO−3+ 10 e−+ 12 H+→ N2+ 6 H2O
- Thecombustionofhydrocarbons,such as in aninternal combustion engine,produceswater,carbon dioxide,some partially oxidized forms such ascarbon monoxide,and heatenergy.Complete oxidation of materials containingcarbonproduces carbon dioxide.
- The stepwise oxidation of a hydrocarbon by oxygen, inorganic chemistry,produces water and, successively: analcohol,analdehydeor aketone,acarboxylic acid,and then aperoxide.
Corrosion and rusting
[edit]

- The termcorrosionrefers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen.Rusting,the formation ofiron oxides,is a well-known example of electrochemical corrosion: it forms as a result of the oxidation ofironmetal. Common rust often refers toiron(III) oxide,formed in the following chemical reaction:
- 4 Fe + 3 O2→ 2 Fe2O3
- The oxidation of iron(II) to iron(III) byhydrogen peroxidein the presence of anacid:
- Fe2+→ Fe3++ e−
- H2O2+ 2 e−→ 2 OH−
- Here the overall equation involves adding the reduction equation to twice the oxidation equation, so that the electrons cancel:
- 2 Fe2++ H2O2+ 2 H+→ 2 Fe3++ 2 H2O
Disproportionation
[edit]Adisproportionationreaction is one in which a single substance is both oxidized and reduced. For example,thiosulfateion with sulfur in oxidation state +2 can react in the presence of acid to form elemental sulfur (oxidation state 0) andsulfur dioxide(oxidation state +4).
- S2O2−3+ 2 H+→ S + SO2+ H2O
Thus one sulfur atom is reduced from +2 to 0, while the other is oxidized from +2 to +4.[8]: 176
Redox reactions in industry
[edit]Cathodic protectionis a technique used to control the corrosion of a metal surface by making it thecathodeof anelectrochemical cell.A simple method of protection connects protected metal to a more easily corroded "sacrificial anode"to act as theanode.The sacrificial metal, instead of the protected metal, then corrodes. A common application of cathodic protection is ingalvanizedsteel, in which a sacrificial zinc coating on steel parts protects them from rust.[citation needed]
Oxidation is used in a wide variety of industries, such as in the production ofcleaning productsand oxidizingammoniato producenitric acid.[citation needed]
Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or supportelectrosynthesis.Metaloresoften contain metals in oxidized states, such as oxides or sulfides, from which the pure metals are extracted bysmeltingat high temperatures in the presence of a reducing agent. The process ofelectroplatinguses redox reactions to coat objects with a thin layer of a material, as inchrome-platedautomotiveparts,silver platingcutlery,galvanizationandgold-platedjewelry.[citation needed]
Redox reactions in biology
[edit]
Many essentialbiologicalprocesses involve redox reactions. Before some of these processes can begin iron must beassimilatedfrom the environment.[24]
Cellular respiration,for instance, is the oxidation ofglucose(C6H12O6) toCO2and the reduction ofoxygentowater.The summary equation for cell respiration is:
- C6H12O6+ 6 O2→ 6 CO2+ 6 H2O + Energy
The process of cell respiration also depends heavily on the reduction ofNAD+to NADH and the reverse reaction (the oxidation of NADH to NAD+).Photosynthesisand cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2+ 6 H2O +light energy→ C6H12O6+ 6 O2
Biological energyis frequently stored and released using redox reactions. Photosynthesis involves the reduction ofcarbon dioxideintosugarsand the oxidation ofwaterinto molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reducenicotinamide adenine dinucleotide(NAD+) to NADH, which then contributes to the creation of aproton gradient,which drives the synthesis ofadenosine triphosphate(ATP) and is maintained by the reduction of oxygen. In animal cells,mitochondriaperform similar functions.
Free radicalreactions are redox reactions that occur as part ofhomeostasisand killingmicroorganisms.In these reactions, an electron detaches from a molecule and then re-attaches almost instantaneously. Free radicals are part of redox molecules and can become harmful to the human body if they do not reattach to the redox molecule or anantioxidant.
The term redox state is often used to describe the balance ofGSH/GSSG,NAD+/NADH andNADP+/NADPHin a biological system such as acellororgan.The redox state is reflected in the balance of several sets of metabolites (e.g.,lactateandpyruvate,beta-hydroxybutyrate,andacetoacetate), whose interconversion is dependent on these ratios. Redox mechanisms also control some cellular processes. Redox proteins and their genes must be co-located for redox regulation according to theCoRR hypothesisfor the function ofDNAinmitochondriaandchloroplasts.
Redox cycling
[edit]Wide varieties ofaromatic compoundsareenzymaticallyreduced to formfree radicalsthat contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety offlavoenzymesand theircoenzymes.Once formed, these anion free radicals reduce molecular oxygen tosuperoxideand regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as afutile cycleor redox cycling.
Redox reactions in geology
[edit]
Minerals are generally oxidized derivatives of metals. Iron is mined as itsmagnetite(Fe3O4). Titanium is mined as its dioxide, usually in the form ofrutile(TiO2). These oxides must be reduced to obtain the corresponding metals, often achieved by heating these oxides with carbon or carbon monoxide as reducing agents.Blast furnacesare the reactors where iron oxides and coke (a form of carbon) are combined to produce molten iron. The main chemical reaction producing the molten iron is:[25]
- Fe2O3+ 3 CO → 2 Fe + 3 CO2
Redox reactions in soils
[edit]Electron transferreactions are central to myriad processes and properties in soils, andredox potential,quantified as Eh (platinum electrode potential (voltage) relative to the standard hydrogen electrode) or pe (analogous to pH as -log electron activity), is a master variable, along with pH, that controls and is governed by chemical reactions and biological processes. Early theoretical research with applications to flooded soils andpaddy riceproduction was seminal for subsequent work on thermodynamic aspects of redox and plant root growth in soils.[26]Later work built on this foundation, and expanded it for understanding redox reactions related to heavy metal oxidation state changes,pedogenesisand morphology, organic compound degradation and formation,free radicalchemistry,wetlanddelineation,soil remediation,and various methodological approaches for characterizing the redox status of soils.[27][28]
Mnemonics
[edit]The key terms involved in redox can be confusing.[29][30]For example, a reagent that is oxidized loses electrons; however, that reagent is referred to as the reducing agent. Likewise, a reagent that is reduced gains electrons and is referred to as the oxidizing agent.[31]Thesemnemonicsare commonly used by students to help memorise the terminology:[32]
- "OIL RIG"—oxidationisloss of electrons,reductionisgain of electrons[29][30][31][32]
- "LEO the lion says GER [grr]" —loss ofelectrons isoxidation,gain ofelectrons isreduction[29][30][31][32]
- "LEORA says GEROA" — the loss of electrons is called oxidation (reducing agent); the gain of electrons is called reduction (oxidizing agent).[31]
- "RED CAT" and "AN OX", or "AnOx RedCat" ( "an ox-red cat" ) — reduction occurs at the cathode and the anode is for oxidation
- "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons)
- "PANIC" – Positive Anode and Negative is Cathode. This applies toelectrolytic cellswhich release stored electricity, and can be recharged with electricity. PANIC does not apply to cells that can be recharged with redox materials. Thesegalvanic or voltaic cells,such asfuel cells,produce electricity from internal redox reactions. Here, the positive electrode is the cathode and the negative is the anode.
See also
[edit]- Anaerobic respiration
- Bessemer process
- Bioremediation
- Calvin cycle
- Chemical equation
- Chemical looping combustion
- Citric acid cycle
- Electrochemical series
- Electrochemistry
- Electrolysis
- Electron equivalent
- Electron transport chain
- Electrosynthesis
- Galvanic cell
- Hydrogenation
- Membrane potential
- Microbial fuel cell
- Murburn concept
- Nucleophilic abstraction
- Organic redox reaction
- Oxidative addition and reductive elimination
- Oxidative phosphorylation
- Partial oxidation
- Pro-oxidant
- Redox gradient
- Redox potential
- Redox therapy
- Reducing agent
- Reducing atmosphere
- Reduction potential
- Thermic reaction
- Transmetalation
- Sulfur cycle
References
[edit]- ^"Metals".Bitesize.BBC.Archivedfrom the original on November 3, 2022.
- ^"redox – definition of redox in English | Oxford Dictionaries".Oxford Dictionaries | English.Archived fromthe originalon October 1, 2017.RetrievedMay 15,2017.
- ^abcdePetrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002).General Chemistry(8th ed.). Prentice-Hall.ISBN0-13-014329-4.
- ^"Redox Reactions".wiley.com.Archivedfrom the original on May 30, 2012.RetrievedMay 9,2012.
- ^abcHaustein, Catherine Hinga (2014)."Oxidation-reduction reaction".In K. Lee Lerner; Brenda Wilmoth Lerner (eds.).The Gale Encyclopedia of Science(5th ed.). Farmington Hills, MI: Gale Group.
- ^Harper, Douglas."redox".Online Etymology Dictionary.
- ^Pingarrón, José M.; Labuda, Ján; Barek, Jiří; Brett, Christopher M. A.; Camões, Maria Filomena; Fojta, Miroslav; Hibbert, D. Brynn (2020)."Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019)".Pure and Applied Chemistry.92(4): 641–694.doi:10.1515/pac-2018-0109.
- ^abcdPetrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2017).General Chemistry: Principles and Modern applications(11th ed.). Toronto: Pearson.ISBN978-0-13-293128-1.
- ^"Nitric Acid Fact Sheet"(PDF).Department of Environmental Safety, Sustainability & Risk.University of Maryland.RetrievedFebruary 12,2024.
- ^abWhitten, Kenneth W.; Gailey, Kenneth D.; Davis, Raymond E. (1992).General Chemistry(4th ed.). Saunders College Publishin. p. 147.ISBN0-03-072373-6.
- ^Jain JL (2004).Fundamentals of Biochemistry.S. Chand.ISBN81-219-2453-7.
- ^Lehninger AL, Nelson DL, Cox MM (January 1, 2017).Lehninger Principles of Biochemistry(Seventh ed.). New York, NY.ISBN9781464126116.OCLC986827885.
{{cite book}}:CS1 maint: location missing publisher (link) - ^https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch19/oxred_3.php#top
- ^Hudlický, Miloš (1996).Reductions in Organic Chemistry.Washington, D.C.: American Chemical Society. p. 429.ISBN978-0-8412-3344-7.
- ^Hudlický, Miloš (1990).Oxidations in Organic Chemistry.Washington, D.C.: American Chemical Society. pp.456.ISBN978-0-8412-1780-5.
- ^Bockris, John O'M.; Reddy, Amulya K. N. (1970).Modern Electrochemistry.Plenum Press. pp. 352–3.
- ^Bockris, John O'M.; Reddy, Amulya K.N. (2013) [1970].Modern Electrochemistry.Vol. 1. Springer Science & Business Media. p. 494.ISBN9781461574675.RetrievedMarch 29,2020.
The homogeneous proton-transfer reactions described are similar to homogeneous electron-transfer reactions in that the overall electron-transfer reaction can be decomposed into one electronation reaction and one deelectronation reaction.
- ^IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book" ). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk.ISBN0-9678550-9-8.https://goldbook.iupac.org/terms/view/R05222
- ^IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book" ). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk.ISBN0-9678550-9-8.https://goldbook.iupac.org/terms/view/O04362
- ^Mailloux, Ryan J. (April 2015)."Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species".Redox Biology.4:381–398.doi:10.1016/j.redox.2015.02.001.PMC4348434.PMID25744690.
- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "Inner-sphere electron transfer".doi:10.1351/goldbook.I03052
- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "Outer-sphere electron transfer".doi:10.1351/goldbook.O04351
- ^"Bond Energy and Enthalpy".
- ^"Titles of Volumes 1–44 in the Metal Ions in Biological Systems Series".Metals, Microbes, and Minerals - the Biogeochemical Side of Life.De Gruyter. 2021. pp. xxiii–xxiv.doi:10.1515/9783110589771-005.ISBN9783110588903.S2CID242013948.
- ^Oeters, Franz; Ottow, Manfred; Meiler, Heinrich; Lüngen, Hans Bodo; Koltermann, Manfred; Buhr, Andreas; Yagi, Jun-Ichiro; Formanek, Lothar; Rose, Fritz; Flickenschild, Jürgen; Hauk, Rolf; Steffen, Rolf; Skroch, Reiner; Mayer-Schwinning, Gernot; Bünnagel, Heinz-Lothar; Hoff, Hans-Georg (2006). "Iron".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a14_461.pub2.ISBN978-3527306732.
- ^Ponnamperuma, F.N. (1972).The Chemistry of Submerged Soils.Advances in Agronomy. Vol. 24. pp. 29–96.doi:10.1016/S0065-2113(08)60633-1.ISBN978-0-12-000724-0.
- ^Bartlett, Richmond J.; James, Bruce R. (1991). "Redox chemistry of soils".Advances in Agronomy.39:151–208.
- ^James, Bruce R.; Brose, Dominic A. (2012). "Oxidation-reduction phenomena". In Huang, Pan Ming; Li, Yuncong; Sumner, Malcolm E. (eds.).Handbook of soil sciences: properties and processes(second ed.). Boca Raton, Florida:CRC Press.pp. 14-1 -- 14-24.ISBN978-1-4398-0305-9.
- ^abcRobertson, William (2010).More Chemistry Basics.National Science Teachers Association. p. 82.ISBN978-1-936137-74-9.
- ^abcPhillips, John; Strozak, Victor; Wistrom, Cheryl (2000).Chemistry: Concepts and Applications.Glencoe McGraw-Hill. p. 558.ISBN978-0-02-828210-7.
- ^abcdRodgers, Glen (2012).Descriptive Inorganic, Coordination, and Solid-State Chemistry.Brooks/Cole, Cengage Learning. p. 330.ISBN978-0-8400-6846-0.
- ^abcZumdahl, Steven; Zumdahl, Susan (2009).Chemistry.Houghton Mifflin. p. 160.ISBN978-0-547-05405-6.
Further reading
[edit]- Schüring, J.; Schulz, H. D.; Fischer, W. R.; Böttcher, J.; Duijnisveld, W. H., eds. (1999).Redox: Fundamentals, Processes and Applications.Heidelberg: Springer-Verlag. p. 246.hdl:10013/epic.31694.d001.ISBN978-3-540-66528-1.
- Tratnyek, Paul G.; Grundl, Timothy J.; Haderlein, Stefan B., eds. (2011).Aquatic Redox Chemistry.ACS Symposium Series. Vol. 1071.doi:10.1021/bk-2011-1071.ISBN978-0-8412-2652-4.