Reelin
Reelin,encoded by theRELNgene,[5]is a large secretedextracellular matrixglycoproteinthat helps regulate processes ofneuronal migrationand positioning in the developing brain by controllingcell–cell interactions.Besides this important role in earlydevelopment,reelin continues to work in the adult brain.[6]It modulatessynaptic plasticityby enhancing the induction and maintenance oflong-term potentiation.[7][8]It also stimulates dendrite[9]anddendritic spine[10]development and regulates the continuing migration ofneuroblastsgenerated inadult neurogenesissites like thesubventricularandsubgranular zones.It is found not only in thebrainbut also in theliver,thyroid gland,adrenal gland,fallopian tube,breastand in comparatively lower levels across a range of anatomical regions.[11]
Reelin has been suggested to be implicated in pathogenesis of several brain diseases. The expression of the protein has been found to be significantly lower inschizophreniaand psychoticbipolar disorder,[12]but the cause of this observation remains uncertain, as studies show thatpsychotropic medication itself affects reelin expression.Moreover,epigenetichypotheses aimed at explaining the changed levels of reelin expression[13]are controversial.[14][15]Total lack of reelin causes a form oflissencephaly.Reelin may also play a role inAlzheimer's disease,[16]temporal lobe epilepsyandautism.
Reelin's name comes from the abnormal reelinggaitofreelermice,[17]which were later found to have a deficiency of this brainproteinand werehomozygousfor mutation of the RELN gene. The primary phenotype associated with loss of reelin function is a failure of neuronal positioning throughout the developingcentral nervous system(CNS). The miceheterozygousfor the reelin gene, while having little neuroanatomical defects, display theendophenotypictraits linked to psychotic disorders.[18]
Discovery
[edit]
Mutant mice have provided insight into the underlying molecular mechanisms of the development of thecentral nervous system.Useful spontaneous mutations were first identified by scientists who were interested inmotor behavior,and it proved relatively easy to screenlittermatesfor mice that showed difficulties moving around the cage. A number of such mice were found and given descriptive names such as reeler, weaver, lurcher, nervous, and staggerer.[citation needed]
The "reeler"mouse was described for the first time in 1951 byD.S.FalconerinEdinburgh Universityas a spontaneous variant arising in a colony of at least mildly inbred snowy-white bellied mice stock in 1948.[17]Histopathologicalstudies in the 1960s revealed that thecerebellumof reeler mice is dramatically decreased in size while the normal laminar organization found in several brain regions is disrupted.[19]The 1970s brought about the discovery of cellular layer inversion in the mouse neocortex,[20]which attracted more attention to the reeler mutation.
In 1994, a newalleleof reeler was obtained by means of insertionalmutagenesis.[21]This provided the firstmolecular markerof thelocus,permitting the RELN gene to be mapped to chromosome 7q22 and subsequently cloned and identified.[22]Japanese scientists atKochi Medical Schoolsuccessfully raised antibodies against normal brain extracts in reeler mice, later these antibodies were found to be specificmonoclonal antibodiesfor reelin, and were termed CR-50 (Cajal-Retzius marker 50).[23]They noted that CR-50 reacted specifically withCajal-Retzius neurons,whose functional role was unknown until then.[citation needed]
The Reelin receptors,apolipoprotein E receptor 2(ApoER2) andvery-low-density lipoprotein receptor(VLDLR), were discovered by Trommsdorff, Herz and colleagues, who initially found that the cytosolic adaptor protein Dab1 interacts with the cytoplasmic domain of LDL receptor family members.[24]They then went on to show that the doubleknockoutmice for ApoER2 and VLDLR, which both interact with Dab1, had cortical layering defects similar to those in reeler.[25]
Thedownstreampathwayof reelin was further clarified with the help of other mutant mice, includingyotariandscrambler.These mutants have phenotypes similar to that of reeler mice, but without mutation in reelin. It was then demonstrated that the mousedisabled homologue 1(Dab1) gene is responsible for the phenotypes of these mutant mice, as Dab1 protein was absent (yotari) or only barely detectable (scrambler) in these mutants.[26]Targeted disruption of Dab1 also caused a phenotype similar to that of reeler. Pinpointing theDAB1as a pivotal regulator of the reelin signaling cascade started the tedious process of deciphering its complex interactions.[citation needed]
There followed a series of speculative reports linking reelin's genetic variation and interactions to schizophrenia, Alzheimer's disease, autism and other highly complex dysfunctions. These and other discoveries, coupled with the perspective of unraveling the evolutionary changes that allowed for the creation of human brain, highly intensified the research. As of 2008, some 13 years after the gene coding the protein was discovered, hundreds of scientific articles address the multiple aspects of its structure and functioning.[27][28]
Tissue distribution and secretion
[edit]Studies show that reelin is absent fromsynaptic vesiclesand is secreted viaconstitutive secretory pathway,being stored inGolgisecretory vesicles.[29]Reelin's release rate is not regulated bydepolarization,but strictly depends on its synthesis rate. This relationship is similar to that reported for the secretion of otherextracellular matrixproteins.[citation needed]
During the brain development, reelin is secreted in the cortex and hippocampus by the so-calledCajal-Retzius cells,Cajal cells, and Retzius cells.[30]Reelin-expressing cells in the prenatal and early postnatal brain are predominantly found in the marginal zone (MZ) of the cortex and in the temporarysubpial granular layer(SGL), which is manifested to the highest extent in human,[31]and in the hippocampalstratum lacunosum-moleculareand the upper marginal layer of thedentate gyrus.
In the developingcerebellum,reelin is expressed first in the externalgranule celllayer (EGL), before the granule cell migration to the internal granule cell layer (IGL) takes place.[32]
Having peaked just after the birth, the synthesis of reelin subsequently goes down sharply, becoming more diffuse compared with the distinctly laminar expression in the developing brain. In the adult brain, reelin is expressed byGABA-ergicinterneuronsof the cortex and glutamatergic cerebellar neurons,[33]the glutamatergic stellate cells and fan cells in the superficialentorhinal cortexthat are supposed to carry a role in encoding newepisodic memories,[34]and by the few extant Cajal-Retzius cells. Among GABAergic interneurons, reelin seems to be detected predominantly in those expressingcalretininandcalbindin,likebitufted,horizontal,andMartinotti cells,but notparvalbumin-expressing cells, likechandelierorbasket neurons.[35][36]In the white matter, a minute proportion ofinterstitial neuronshas also been found to stain positive for reelin expression.[37]

Outside the brain, reelin is found in adult mammalian blood,liver,pituitarypars intermedia,and adrenalchromaffin cells.[38]In the liver, reelin is localized inhepatic stellate cells.[39]The expression of reelin increases when the liver is damaged, and returns to normal following its repair.[40] In the eyes, reelin is secreted byretinal ganglion cellsand is also found in theendothelial layer of the cornea.[41]Just as in the liver, its expression increases after an injury has taken place.[citation needed]
The protein is also produced by theodontoblasts,which are cells at the margins of the dental pulp. Reelin is found here both during odontogenesis and in the mature tooth.[42]Some authors suggest that odontoblasts play an additional role as sensory cells able totransducepain signals to the nerve endings.[43]According to the hypothesis, reelin participates in the process[28]by enhancing the contact between odontoblasts and the nerve terminals.[44]
Structure
[edit]
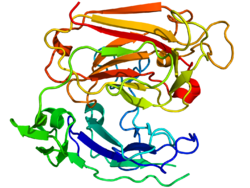
Reelin is composed of 3461 amino acids with a relative molecular mass of 388kDa.It also hasserine proteaseactivity.[46]Murine RELN gene consists of 65exonsspanning approximately 450kb.[47]One exon, coding for only two amino acids near the protein'sC-terminus,undergoesalternative splicing,but the exact functional impact of this is unknown.[28]Two transcription initiation sites and two polyadenylation sites are identified in the gene structure.[47]
The reelin protein starts with a signaling peptide 27 amino acids in length, followed by a region bearing similarity toF-spondin(thereeler domain), marked as "SP" on the scheme, and by a region unique to reelin, marked as "H". Next comes 8 repeats of 300–350 amino acids. These are calledreelin repeatsand have anepidermal growth factormotif at their center, dividing each repeat into two subrepeats,A(theBNR/Asp-box repeat) andB(theEGF-like domain). Despite this interruption, the two subdomains make direct contact, resulting in a compact overall structure.[48]
The final reelin domain contains a highly basic and short C-terminal region (CTR, marked "+" ) with a length of 32 amino acids. This region is highly conserved, being 100% identical in all investigated mammals. It was thought that CTR is necessary for reelin secretion, because the Orleansreelermutation, which lacks a part of 8th repeat and the whole CTR, is unable to secrete the misshaped protein, leading to its concentration in cytoplasm. However, other studies have shown that the CTR is not essential for secretion itself, but mutants lacking the CTR were much less efficient in activating downstream signaling events.[49]
Reelin is cleavedin vivoat two sites located after domains 2 and 6 – approximately between repeats 2 and 3 and between repeats 6 and 7, resulting in the production of three fragments.[50]This splitting does not decrease the protein's activity, as constructs made of the predicted central fragments (repeats 3–6) bind to lipoprotein receptors, triggerDab1phosphorylationand mimic functions of reelin duringcortical platedevelopment.[51]Moreover, the processing of reelin by embryonic neurons may be necessary for proper corticogenesis.[52]
Function
[edit]
The primary functions of Reelin are the regulation of corticogenesis and neuronal cell positioning in the prenatal period, but the protein also continues to play a role in adults. Reelin is found in numerous tissues and organs, and one could roughly subdivide its functional roles by the time of expression and by localisation of its action.[11]
During development
[edit]A number of non-nervous tissues and organs express reelin during development, with the expression sharply going down after organs have been formed. The role of the protein here is largely unexplored, because the knockout mice show no major pathology in these organs. Reelin's role in the growing central nervous system has been extensively characterized. It promotes the differentiation of progenitor cells intoradial gliaand affects the orientation of its fibers, which serve as the guides for the migrating neuroblasts.[55]The position of reelin-secreting cell layer is important, because the fibers orient themselves in the direction of its higher concentration.[56]For example, reelin regulates the development of layer-specific connections in hippocampus and entorhinal cortex.[57][58]

Mammaliancorticogenesisis another process where reelin plays a major role. In this process the temporary layer called preplate is split into the marginal zone on the top and subplate below, and the space between them is populated by neuronal layers in the inside-out pattern. Such an arrangement, where the newly created neurons pass through the settled layers and position themselves one step above, is a distinguishing feature of mammalian brain, in contrast to the evolutionary older reptile cortex, in which layers are positioned in an "outside-in" fashion. When reelin is absent, like in the mutantreelermouse, the order of cortical layering becomes roughly inverted, with younger neurons finding themselves to be unable to pass the settled layers. Subplate neurons fail to stop and invade the upper most layer, creating the so-called superplate in which they mix withCajal-Retzius cellsand some cells normally destined for the second layer.[citation needed]

There is no agreement concerning the role of reelin in the proper positioning of cortical layers. The original hypothesis, that the protein is a stop signal for the migrating cells, is supported by its ability to induce the dissociation,[59]its role in asserting the compact granule cell layer in the hippocampus, and by the fact that migrating neuroblasts evade the reelin-rich areas. But an experiment in which murine corticogenesis went normally despite the malpositioned reelin secreting layer,[60]and lack of evidence that reelin affects the growth cones and leading edges of neurons, caused some additional hypotheses to be proposed. According to one of them, reelin makes the cells more susceptible to some yet undescribed positional signaling cascade.[citation needed]
Reelin may also ensure correct neuronal positioning in thespinal cord:according to one study, location and level of its expression affects the movement of sympathetic preganglionic neurons.[61]
The protein is thought to act on migrating neuronal precursors and thus controls correct cell positioning in the cortex and other brain structures. The proposed role is one of a dissociation signal for neuronal groups, allowing them to separate and go from tangential chain-migration to radial individual migration.[59]Dissociation detaches migrating neurons from theglial cellsthat are acting as their guides, converting them into individual cells that can strike out alone to find their final position.[citation needed]

Reelin takes part in the developmental change ofNMDA receptorconfiguration, increasing mobility ofNR2B-containing receptors and thus decreasing the time they spend at thesynapse.[63][dead link][64][65]It has been hypothesized that this may be a part of the mechanism behind the "NR2B-NR2A switch" that is observed in the brain during its postnatal development.[66]Ongoing reelin secretion by GABAergic hippocampal neurons is necessary to keep NR2B-containing NMDA receptors at a low level.[62]
In adults
[edit]In the adult nervous system, reelin plays an eminent role at the two most active neurogenesis sites, the subventricular zone and the dentate gyrus. In some species, the neuroblasts from the subventricular zone migrate in chains in therostral migratory stream(RMS) to reach the olfactory bulb, where reelin dissociates them into individual cells that are able to migrate further individually. They change their mode of migration from tangential to radial, and begin using the radial glia fibers as their guides. There are studies showing that along the RMS itself the two receptors,ApoER2andVLDLR,and their intracellular adapterDAB1function independently of Reelin,[67]most likely by the influence of a newly proposed ligand,thrombospondin-1.[53]In the adult dentate gyrus, reelin provides guidance cues for new neurons that are constantly arriving to the granule cell layer from subgranular zone, keeping the layer compact.[68]
Reelin also plays an important role in the adult brain by modulating cortical pyramidal neurondendritic spineexpression density, the branching ofdendrites,and the expression oflong-term potentiation[8]as its secretion is continued diffusely by the GABAergic cortical interneurons those origin is traced to the medialganglionic eminence.
In the adult organism the non-neural expression is much less widespread, but goes up sharply when some organs are injured.[40][41]The exact function of reelin upregulation following an injury is still being researched.[citation needed]
Evolutionary significance
[edit]

Reelin-DAB1 interactions could have played a key role in the structural evolution of the cortex that evolved from a single layer in the common predecessor of theamniotesinto multiple-layered cortex of contemporary mammals.[69]Research shows that reelin expression goes up as the cortex becomes more complex, reaching the maximum in the human brain in which the reelin-secreting Cajal-Retzius cells have significantly more complex axonal arbour.[70]Reelin is present in the telencephalon of all the vertebrates studied so far, but the pattern of expression differs widely. For example,zebrafishhave no Cajal-Retzius cells at all; instead, the protein is being secreted by other neurons.[71][72]These cells do not form a dedicated layer in amphibians, and radial migration in their brains is very weak.[71]
As the cortex becomes more complex and convoluted, migration along the radial glia fibers becomes more important for the proper lamination. The emergence of a distinct reelin-secreting layer is thought to play an important role in this evolution.[56]There are conflicting data concerning the importance of this layer,[60]and these are explained in the literature either by the existence of an additional signaling positional mechanism that interacts with the reelin cascade,[60]or by the assumption that mice that are used in such experiments have redundant secretion of reelin[73]compared with more localized synthesis in the human brain.[31]
Cajal-Retzius cells, most of which disappear around the time of birth, coexpress reelin with theHAR1gene that is thought to have undergone the most significant evolutionary change in humans compared with chimpanzee, being the most "evolutionary accelerated" of the genes from thehuman accelerated regions.[74]There is also evidence of that variants in the DAB1 gene have been included in a recent selective sweep in Chinese populations.[75][76]
Mechanism of action
[edit]
SFK:Src family kinases.
JIP:JNK-interacting protein 1
Receptors
[edit]Reelin's control of cell-cell interactions is thought to be mediated by binding of reelin to the two members oflow density lipoprotein receptor gene family:VLDLRand theApoER2.[78][79][80][81]The two main reelin receptors seem to have slightly different roles: VLDLR conducts the stop signal, while ApoER2 is essential for the migration of late-born neocortical neurons.[82]It also has been shown that the N-terminal region of reelin, a site distinct from the region of reelin shown to associate with VLDLR/ApoER2 binds to the alpha-3-beta-1integrinreceptor.[83]The proposal that the protocadherinCNR1 behaves as a Reelin receptor[84]has been disproven.[51]
As members of lipoprotein receptor superfamily, both VLDLR and ApoER2 have in their structure an internalization domain calledNPxYmotif.After binding to the receptors reelin is internalized byendocytosis,and the N-terminal fragment of the protein is re-secreted.[85]This fragment may serve postnatally to prevent apical dendrites of cortical layer II/III pyramidal neurons from overgrowth, acting via a pathway independent of canonical reelin receptors.[86]
Reelin receptors are present on bothneuronsandglial cells.Furthermore,radial gliaexpress the same amount ofApoER2but being ten times less rich inVLDLR.[55]beta-1 integrin receptorson glial cells play more important role in neuronal layering than the same receptors on the migrating neuroblasts.[87]
Reelin-dependent strengthening oflong-term potentiationis caused byApoER2interaction withNMDA receptor.This interaction happens when ApoER2 has a region coded by exon 19. ApoER2 gene is alternatively spliced, with the exon 19-containing variant more actively produced during periods of activity.[88]According to one study, the hippocampal reelin expression rapidly goes up when there is need to store a memory, asdemethylasesopen up the RELN gene.[89]The activation of dendrite growth by reelin is apparently conducted throughSrcfamilykinasesand is dependent upon the expression ofCrkfamily proteins,[90]consistent with the interaction of Crk and CrkL with tyrosine-phosphorylated Dab1.[91]Moreover, aCre-loxP recombinationmouse model that lacksCrkandCrkLin most neurons[92]was reported to have thereelerphenotype, indicating that Crk/CrkL lie betweenDAB1andAktin the reelin signaling chain.
Signaling cascades
[edit]Reelin activates the signaling cascade ofNotch-1,inducing the expression ofFABP7and prompting progenitor cells to assumeradial glialphenotype.[93]In addition, corticogenesisin vivois highly dependent upon reelin being processed by embryonic neurons,[52]which are thought to secrete some as yet unidentifiedmetalloproteinasesthat free the central signal-competent part of the protein. Some other unknown proteolytic mechanisms may also play a role.[94]It is supposed that full-sized reelin sticks to the extracellular matrix fibers on the higher levels, and the central fragments, as they are being freed up by the breaking up of reelin, are able to permeate into the lower levels.[52]It is possible that asneuroblastsreach the higher levels they stop their migration either because of the heightened combined expression of all forms of reelin, or due to the peculiar mode of action of the full-sized reelin molecules and its homodimers.[28]
The intracellular adaptorDAB1binds to the VLDLR and ApoER2 through anNPxYmotif and is involved in transmission of Reelin signals through these lipoprotein receptors. It becomes phosphorylated bySrc[95]andFyn[96]kinases and apparently stimulates theactincytoskeleton to change its shape, affecting the proportion of integrin receptors on the cell surface, which leads to the change inadhesion.Phosphorylation of DAB1 leads to itsubiquitinationand subsequent degradation, and this explains the heightened levels of DAB1 in the absence of reelin.[97]Suchnegative feedbackis thought to be important for proper cortical lamination.[98]Activated by two antibodies, VLDLR and ApoER2 cause DAB1 phosphorylation but seemingly without the subsequent degradation and without rescuing thereelerphenotype, and this may indicate that a part of the signal is conducted independently of DAB1.[51]

A protein having an important role inlissencephalyand accordingly calledLIS1(PAFAH1B1), was shown to interact with the intracellular segment of VLDLR, thus reacting to the activation of reelin pathway.[77]
Complexes
[edit]Reelin molecules have been shown[99][100]to form a large protein complex, adisulfide-linkedhomodimer.If the homodimer fails to form, efficient tyrosinephosphorylationof DAB1in vitrofails. Moreover, the two main receptors of reelin are able to form clusters[101]that most probably play a major role in the signaling, causing the intracellular adaptor DAB1 to dimerize or oligomerize in its turn. Such clustering has been shown in the study to activate the signaling chain even in the absence of Reelin itself.[101]In addition, reelin itself can cut the peptide bonds holding other proteins together, being aserine protease,[46]and this may affect the cellular adhesion and migration processes. Reelin signaling leads to phosphorylation ofactin-interacting proteincofilin 1at ser3; this may stabilize the actin cytoskeleton and anchor the leading processes of migrating neuroblasts, preventing their further growth.[102][103]
Interaction with Cdk5
[edit]Cyclin-dependent kinase 5(Cdk5), a major regulator of neuronal migration and positioning, is known to phosphorylateDAB1[104][105][106]and other cytosolic targets of reelin signaling, such asTau,[107]which could be activated also via reelin-induced deactivation ofGSK3B,[108]andNUDEL,[109]associated withLis1,one of the DAB1 targets.LTPinduction by reelin in hippocampal slices fails inp35knockouts.[110]P35 is a key Cdk5 activator, and double p35/Dab1, p35/RELN, p35/ApoER2, p35/VLDLR knockouts display increased neuronal migration deficits,[110][111]indicating a synergistic action of reelin → ApoER2/VLDLR → DAB1 and p35/p39 → Cdk5 pathways in the normal corticogenesis.
Possible pathological role
[edit]Lissencephaly
[edit]Disruptions of the RELN gene are considered to be the cause of the rare form oflissencephalywithcerebellar hypoplasiaclassed as amicrolissencephalycalledNorman-Roberts syndrome.[112][113]The mutations disruptsplicingof the RELNmRNAtranscript, resulting in low or undetectable amounts of reelin protein. Thephenotypein these patients was characterized byhypotonia,ataxia,and developmental delay, with lack of unsupported sitting and profound mental retardation with little or no language development. Seizures andcongenital lymphedemaare also present. A novelchromosomal translocationcausing the syndrome was described in 2007.[114]
Schizophrenia
[edit]Reduced expression of reelin and itsmRNAlevels in the brains ofschizophreniasufferers had been reported in 1998[115]and 2000,[116]and independently confirmed in postmortem studies of the hippocampus,[12]cerebellum,[117]basal ganglia,[118]and cerebral cortex.[119][120]The reduction may reach up to 50% in some brain regions and is coupled with reduced expression ofGAD-67enzyme,[117]which catalyses the transition ofglutamatetoGABA.Blood levelsof reelin and itsisoformsare also altered in schizophrenia, along withmood disorders,according to one study.[121]Reduced reelin mRNA prefrontal expression in schizophrenia was found to be the most statistically relevant disturbance found in the multicenter study conducted in 14 separate laboratories in 2001 by Stanley Foundation Neuropathology Consortium.[122]
Epigenetichypermethylation of DNA in schizophrenia patients is proposed as a cause of the reduction,[123][124]in agreement with the observations dating from the 1960s that administration ofmethionineto schizophrenic patients results in a profound exacerbation of schizophrenia symptoms in sixty to seventy percent of patients.[125][126][127][128]The proposed mechanism is a part of the "epigenetic hypothesis for schizophrenia pathophysiology" formulated by a group of scientists in 2008 (D. Grayson; A. Guidotti;E. Costa).[13][129]A postmortem study comparing aDNA methyltransferase(DNMT1) and Reelin mRNA expression in cortical layers I and V of schizophrenic patients and normal controls demonstrated that in the layer V both DNMT1 and Reelin levels were normal, while in the layer I DNMT1 was threefold higher, probably leading to the twofold decrease in the Reelin expression.[130]There is evidence that the change is selective, and DNMT1 is overexpressed in reelin-secreting GABAergic neurons but not in their glutamatergic neighbours.[131][132]Methylationinhibitors andhistone deacetylaseinhibitors, such asvalproic acid,increase reelin mRNA levels,[133][134][135]while L-methionine treatment downregulates the phenotypic expression of reelin.[136]
One study indicated the upregulation of histone deacetylase HDAC1 in the hippocampi of patients.[137]Histone deacetylases suppress gene promoters; hyperacetylation of histones was shown in murine models to demethylate the promoters of both reelin and GAD67.[138]DNMT1 inhibitors in animals have been shown to increase the expression of both reelin and GAD67,[139]and both DNMT inhibitors and HDAC inhibitors shown in one study[140]to activate both genes with comparable dose- and time-dependence. As one study shows,S-adenosyl methionine(SAM) concentration in patients' prefrontal cortex is twice as high as in the cortices of non-affected people.[141]SAM, being a methyl group donor necessary for DNMT activity, could further shift epigenetic control of gene expression.[citation needed]
Chromosome region7q22that harbours theRELNgene is associated with schizophrenia,[142]and the gene itself was associated with the disease in a large study that found the polymorphismrs7341475to increase the risk of the disease in women, but not in men. The women that have thesingle-nucleotide polymorphism(SNP) are about 1.4 times more likely to get ill, according to the study.[143]Allelic variations of RELN have also been correlated with working memory, memory and executive functioning in nuclear families where one of the members suffers from schizophrenia.[142]The association with working memory was later replicated.[144]In one small study, nonsynonymous polymorphismVal997Leuof the gene was associated with left and right ventricular enlargement in patients.[145]
One study showed that patients have decreased levels of one of reelin receptors,VLDLR,in the peripherallymphocytes.[146]After six months ofantipsychotictherapy the expression went up; according to authors, peripheral VLRLR levels may serve as a reliable peripheral biomarker of schizophrenia.[146]
Considering the role of reelin in promoting dendritogenesis,[9][90]suggestions were made that the localized dendritic spine deficit observed in schizophrenia[147][148]could be in part connected with the downregulation of reelin.[149][150]
Reelin pathway could also be linked to schizophrenia and other psychotic disorders through its interaction with risk genes. One example is the neuronal transcription factorNPAS3,disruption of which is linked to schizophrenia[151]and learning disability. Knockout mice lacking NPAS3 or the similar proteinNPAS1have significantly lower levels of reelin;[152]the precise mechanism behind this is unknown. Another example is the schizophrenia-linked geneMTHFR,with murine knockouts showing decreased levels of reelin in the cerebellum.[153]Along the same line, it is worth noting that the gene coding for the subunitNR2Bthat is presumably affected by reelin in the process of NR2B->NR2A developmental change of NMDA receptor composition,[65]stands as one of the strongest riskgene candidates.[154]Another shared aspect between NR2B and RELN is that they both can be regulated by theTBR1transcription factor.[155]
Theheterozygousreeler mouse, which ishaploinsufficientfor the RELN gene, shares several neurochemical and behavioral abnormalities with schizophrenia and bipolar disorder,[156]but the exact relevance of these murine behavioral changes to the pathophysiology of schizophrenia remains debatable.[157]
As previously described, reelin plays a crucial role in modulating early neuroblast migration during brain development. Evidences of altered neural cell positioning in post-mortem schizophrenia patient brains[158][159]and changes togene regulatory networksthat controlcell migration[160][161]suggests a potential link between altered reelin expression in patient brain tissue to disrupted cell migration during brain development. To model the role of reelin in the context of schizophrenia at a cellular level, olfactory neurosphere-derived cells were generated from thenasalbiopsiesof schizophrenia patients, and compared to cells from healthy controls.[160]Schizophrenia patient-derived cells have reduced levels of reelin mRNA[160]and protein[162]when compared to healthy control cells, but expresses the key reelin receptors and DAB1 accessory protein.[162]When grownin vitro,schizophrenia patient-derived cells were unable to respond to reelin coated ontotissue culturesurfaces; In contrast, cells derived from healthy controls were able to alter their cell migration when exposed to reelin.[162]This work went on to show that the lack of cell migration response in patient-derived cells were caused by the cell's inability to produce enoughfocal adhesionsof the appropriate size when in contact with extracellular reelin.[162]More research into schizophrenia cell-based models are needed to look at the function of reelin, or lack of, in the pathophysiology of schizophrenia.
Bipolar disorder
[edit]Decrease in RELN expression with concurrent upregulation ofDNMT1is typical ofbipolar disorderwith psychosis, but is not characteristic of patients with major depression without psychosis, which could speak of specific association of the change with psychoses.[116]One study suggests that unlike in schizophrenia, such changes are found only in the cortex and do not affect the deeper structures in psychotic bipolar patients, as their basal ganglia were found to have the normal levels of DNMT1 and subsequently both the reelin and GAD67 levels were within the normal range.[118]
In a genetic study conducted in 2009, preliminary evidence requiring furtherDNA replicationsuggested that variation of the RELNgene(SNPrs362719) may be associated with susceptibility tobipolar disorderin women.[163]
Autism
[edit]Autismis aneurodevelopmental disorderthat is generally believed to be caused by mutations in several locations, likely triggered by environmental factors. The role of reelin in autism is not decided yet.[164]
Reelin was originally in 2001 implicated in a study finding associations between autism and apolymorphicGGC/CGGrepeatpreceding the 5' ATG initiator codon of the RELN gene in an Italian population. Longer triplet repeats in the 5' region were associated with an increase in autism susceptibility.[165]However, another study of 125 multiple-incidence families and 68 single-incidence families from the subsequent year found no significant difference between the length of the polymorphic repeats in affected and controls. Although, using a family based association test largerreelinalleles were found to be transmitted more frequently than expected to affected children.[166]An additional study examining 158 subjects with German lineage likewise found no evidence of triplet repeat polymorphisms associated with autism.[167]And a larger study from 2004 consisting of 395 families found no association between autistic subjects and the CGG triplet repeat as well as the allele size when compared to age of first word.[168] In 2010 a large study using data from 4 European cohorts would find some evidence for an association between autism and thers362780RELN polymorphism.[169]
Studies oftransgenicmice have been suggestive of an association, but not definitive.[170]
Temporal lobe epilepsy: granule cell dispersion
[edit]Decreased reelin expression in the hippocampal tissue samples from patients withtemporal lobe epilepsywas found to be directly correlated with the extent ofgranule celldispersion (GCD), a major feature of the disease that is noted in 45%–73% of patients.[171][172]The dispersion, according to a small study, is associated with the RELN promoter hypermethylation.[173]According to one study, prolonged seizures in a rat model of mesial temporal lobe epilepsy have led to the loss of reelin-expressing interneurons and subsequent ectopic chain migration and aberrant integration of newborn dentate granule cells. Without reelin, the chain-migrating neuroblasts failed to detach properly.[174]Moreover, in akainate-induced mouse epilepsy model, exogenous reelin had prevented GCD, according to one study.[175]
Alzheimer's disease
[edit]The Reelin receptorsApoER2andVLDLRbelong to theLDLreceptor gene family.[176]All members of this family are receptors forApolipoprotein E(ApoE). Therefore, they are often synonymously referred to as 'ApoE receptors'. ApoE occurs in 3 common isoforms (E2, E3, E4) in the human population.ApoE4is the primary genetic risk factor for late-onsetAlzheimer's disease.This strong genetic association has led to the proposal that ApoE receptors play a central role in the pathogenesis of Alzheimer's disease.[176][177]According to one study, reelin expression andglycosylationpatterns are altered inAlzheimer's disease.In the cortex of the patients, reelin levels were 40% higher compared with controls, but the cerebellar levels of the protein remain normal in the same patients.[178]This finding is in agreement with an earlier study showing the presence of Reelin associated with amyloid plaques in a transgenic AD mouse model.[179]A large genetic study of 2008 showed that RELN gene variation is associated with an increased risk of Alzheimer's disease in women.[180]The number of reelin-producing Cajal-Retzius cells is significantly decreased in the first cortical layer of patients.[181][182]Reelin has been shown to interact withamyloid precursor protein,[183]and, according to one in-vitro study, is able to counteract the Aβ-induced dampening ofNMDA-receptoractivity.[184]This is modulated by ApoE isoforms, which selectively alter the recycling of ApoER2 as well as AMPA and NMDA receptors.[185]
Cancer
[edit]DNA methylationpatterns are often changed in tumours, and the RELN gene could be affected: according to one study, in thepancreatic cancerthe expression is suppressed, along with other reelin pathway components[186]In the same study, cutting the reelin pathway in cancer cells that still expressed reelin resulted in increased motility and invasiveness. On the contrary, inprostate cancerthe RELN expression is excessive and correlates withGleason score.[187]Retinoblastomapresents another example of RELN overexpression.[188]This gene has also been seen recurrently mutated in cases ofacute lymphoblastic leukaemia.[189]
Other conditions
[edit]Onegenome-wide association studyindicates a possible role for RELN gene variation inotosclerosis,an abnormal growth of bone of themiddle ear.[190]In a statistical search for the genes that are differentially expressed in the brains of cerebral malaria-resistant versus cerebral malaria-susceptible mice, Delahaye et al. detected a significant upregulation of both RELN andDAB1and speculated on possible protective effects of such over-expression.[191]In 2020, a study reported a novel variant inRELNgene (S2486G) which was associated withankylosing spondylitisin a large family. This suggested a potential insight into the pathophysiological involvement of reelin via inflammation and osteogenesis pathways in ankylosing spondylitis, and it could broaden the horizon toward new therapeutic strategies.[192]A 2020 study from UT Southwestern Medical Center suggests circulating Reelin levels might correlate with MS severity and stages, and that lowering Reelin levels might be a novel way to treat MS.[193]
Factors affecting reelin expression
[edit]
The expression of reelin is controlled by a number of factors besides the sheer number of Cajal-Retzius cells. For example,TBR1transcription factor regulates RELN along with otherT-element-containing genes.[155]On a higher level, increased maternal care was found to correlate with reelin expression in rat pups; such correlation was reported in hippocampus[195]and in the cortex.[194]According to one report, prolonged exposure tocorticosteronesignificantly decreased reelin expression in murine hippocampi, a finding possibly pertinent to the hypothetical role ofcorticosteroidsindepression.[196]One small postmortem study has found increased methylation of RELN gene in the neocortex of persons past their puberty compared with those that had yet to enter the period of maturation.[197]
Psychotropic medication
[edit]As reelin is being implicated in a number of brain disorders and its expression is usually measured posthumously, assessing the possible medication effects is important.[198]
According to the epigenetic hypothesis, drugs that shift the balance in favour ofdemethylationhave a potential to alleviate the proposed methylation-caused downregulation of RELN and GAD67. In one study, clozapine and sulpiride but not haloperidol and olanzapine were shown to increase the demethylation of both genes in mice pretreated with l-methionine.[199]Valproic acid,ahistone deacetylase inhibitor,when taken in combination with antipsychotics, is proposed to have some benefits. But there are studies conflicting the main premise of the epigenetic hypothesis, and a study by Fatemi et al. shows no increase in RELN expression by valproic acid; that indicates the need for further investigation.[citation needed]
Fatemi et al. conducted the study in which RELN mRNA and reelin protein levels were measured in rat prefrontal cortex following a 21-day ofintraperitoneal injectionsof the following drugs:[28]
| Reelin expression | Clozapine | Fluoxetine | Haloperidol | Lithium | Olanzapine | Valproic Acid |
|---|---|---|---|---|---|---|
| protein | ↓ | ↔ | ↓ | ↓ | ↑ | ↔ |
| mRNA | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ |
In 2009, Fatemi et al. published the more detailed work on rats using the same medication. Here, cortical expression of several participants (VLDLR,DAB1,GSK3B) of the signaling chain was measured besides reelin itself, and also the expression ofGAD65andGAD67.[200]
References
[edit]- ^abcGRCh38: Ensembl release 89: ENSG00000189056–Ensembl,May 2017
- ^abcGRCm38: Ensembl release 89: ENSMUSG00000042453–Ensembl,May 2017
- ^"Human PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Mouse PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"RELN gene".Genetics Home Reference.1 August 2013.Retrieved11 September2022.
- ^Bosch C, Muhaisen A, Pujadas L, Soriano E, Martínez A (2016)."Reelin Exerts Structural, Biochemical and Transcriptional Regulation Over Presynaptic and Postsynaptic Elements in the Adult Hippocampus".Frontiers in Cellular Neuroscience.10:138.doi:10.3389/fncel.2016.00138.PMC4884741.PMID27303269.
- ^Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, et al. (October 2002)."Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning".The Journal of Biological Chemistry.277(42): 39944–52.doi:10.1074/jbc.M205147200.PMID12167620.
- ^abD'Arcangelo G (August 2005)."Apoer2: a reelin receptor to remember".Neuron.47(4): 471–3.doi:10.1016/j.neuron.2005.08.001.PMID16102527.S2CID15091293.
- ^abNiu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G (January 2004)."Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway".Neuron.41(1): 71–84.doi:10.1016/S0896-6273(03)00819-5.PMID14715136.S2CID10716252.
- ^Niu S, Yabut O, D'Arcangelo G (October 2008)."The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons".The Journal of Neuroscience.28(41): 10339–48.doi:10.1523/JNEUROSCI.1917-08.2008.PMC2572775.PMID18842893.
- ^ab"Tissue expression of RELN - Summary - The Human Protein Atlas".www.proteinatlas.org.Retrieved28 May2018.
- ^abFatemi SH, Earle JA, McMenomy T (November 2000)."Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression".Molecular Psychiatry.5(6): 654–63, 571.doi:10.1038/sj.mp.4000783.PMID11126396.
- ^abGrayson DR, Guidotti A, Costa E (17 January 2008)."Current Hypotheses".Schizophrenia Research Forum.schizophreniaforum.org. Archived fromthe originalon 17 September 2008.Retrieved23 August2008.
- ^Tochigi M, Iwamoto K, Bundo M, Komori A, Sasaki T, Kato N, et al. (March 2008). "Methylation status of the reelin promoter region in the brain of schizophrenic patients".Biological Psychiatry.63(5): 530–3.doi:10.1016/j.biopsych.2007.07.003.PMID17870056.S2CID11816759.
- ^Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, et al. (March 2008)."Epigenomic profiling reveals DNA-methylation changes associated with major psychosis".American Journal of Human Genetics.82(3): 696–711.doi:10.1016/j.ajhg.2008.01.008.PMC2427301.PMID18319075.
- ^Kovács KA (December 2021)."Relevance of a Novel Circuit-Level Model of Episodic Memories to Alzheimer's Disease".International Journal of Molecular Sciences.23(1): 462.doi:10.3390/ijms23010462.PMC8745479.PMID35008886.
- ^abFalconer DS (January 1951)."Two new mutants, 'trembler' and 'reeler', with neurological actions in the house mouse (Mus musculus L.)"(PDF).Journal of Genetics.50(2): 192–201.doi:10.1007/BF02996215.PMID24539699.S2CID37918631.
- ^Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E (2006). "Reelin down-regulation in mice and psychosis endophenotypes".Neuroscience and Biobehavioral Reviews.30(8): 1065–77.doi:10.1016/j.neubiorev.2006.04.001.PMID16769115.S2CID21156214.
- ^Hamburgh M (October 1963). "Analysis of the postnatal developmental effects of" reeler, "a neurological mutation in mice. A study in developmental genetics".Developmental Biology.8(2): 165–85.doi:10.1016/0012-1606(63)90040-X.PMID14069672.
- ^Caviness VS (December 1976). "Patterns of cell and fiber distribution in the neocortex of the reeler mutant mouse".The Journal of Comparative Neurology.170(4): 435–47.doi:10.1002/cne.901700404.PMID1002868.S2CID34383977.
- ^Miao GG, Smeyne RJ, D'Arcangelo G, Copeland NG, Jenkins NA, Morgan JI, et al. (November 1994)."Isolation of an allele of reeler by insertional mutagenesis".Proceedings of the National Academy of Sciences of the United States of America.91(23): 11050–4.Bibcode:1994PNAS...9111050M.doi:10.1073/pnas.91.23.11050.PMC45164.PMID7972007.
- ^D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (April 1995). "A protein related to extracellular matrix proteins deleted in the mouse mutant reeler".Nature.374(6524): 719–23.Bibcode:1995Natur.374..719D.doi:10.1038/374719a0.PMID7715726.S2CID4266946.
- ^Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, et al. (May 1995)."The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons".Neuron.14(5): 899–912.doi:10.1016/0896-6273(95)90329-1.PMID7748558.S2CID17993812.
- ^Trommsdorff M, Borg JP, Margolis B, Herz J (December 1998)."Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein".The Journal of Biological Chemistry.273(50): 33556–60.doi:10.1074/jbc.273.50.33556.PMID9837937.
- ^Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, et al. (June 1999)."Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2".Cell.97(6): 689–701.doi:10.1016/S0092-8674(00)80782-5.PMID10380922.S2CID13492626.
- ^Sheldon M, Rice DS, D'Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, et al. (October 1997). "Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice".Nature.389(6652): 730–3.Bibcode:1997Natur.389..730S.doi:10.1038/39601.PMID9338784.S2CID4414738.
- ^"Reelin" mentioned in the titles of scientific literature– a search in theGoogle Scholar
- ^abcdeHossein S. Fatemi, ed. (2008).Reelin Glycoprotein: Structure, Biology and Roles in Health and Disease.Springer. p. 444.ISBN978-0-387-76760-4.
- ^Lacor PN, Grayson DR, Auta J, Sugaya I, Costa E, Guidotti A (March 2000)."Reelin secretion from glutamatergic neurons in culture is independent from neurotransmitter regulation".Proceedings of the National Academy of Sciences of the United States of America.97(7): 3556–61.Bibcode:2000PNAS...97.3556L.doi:10.1073/pnas.050589597.PMC16278.PMID10725375.
- ^Meyer G, Goffinet AM, Fairén A (December 1999). "What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex".Cerebral Cortex.9(8): 765–75.doi:10.1093/cercor/9.8.765.PMID10600995.
- ^abMeyer G, Goffinet AM (July 1998). "Prenatal development of reelin-immunoreactive neurons in the human neocortex".The Journal of Comparative Neurology.397(1): 29–40.doi:10.1002/(SICI)1096-9861(19980720)397:1<29::AID-CNE3>3.3.CO;2-7.PMID9671277.
- ^Schiffmann SN, Bernier B, Goffinet AM (May 1997). "Reelin mRNA expression during mouse brain development".The European Journal of Neuroscience.9(5): 1055–71.doi:10.1111/j.1460-9568.1997.tb01456.x.PMID9182958.S2CID22576790.
- ^Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, et al. (March 1998)."Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats".Proceedings of the National Academy of Sciences of the United States of America.95(6): 3221–6.Bibcode:1998PNAS...95.3221P.doi:10.1073/pnas.95.6.3221.PMC19723.PMID9501244.
- ^Kovács KA (September 2020)."Episodic Memories: How do the Hippocampus and the Entorhinal Ring Attractors Cooperate to Create Them?".Frontiers in Systems Neuroscience.14:68.doi:10.3389/fnsys.2020.559186.PMC7511719.PMID33013334.
- ^Alcántara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, et al. (October 1998)."Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse".The Journal of Neuroscience.18(19): 7779–99.doi:10.1523/JNEUROSCI.18-19-07779.1998.PMC6792998.PMID9742148.
- ^Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ (March 1999)."Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression".Proceedings of the National Academy of Sciences of the United States of America.96(6): 3217–22.Bibcode:1999PNAS...96.3217P.doi:10.1073/pnas.96.6.3217.PMC15922.PMID10077664.
- ^Suárez-Solá ML, González-Delgado FJ, Pueyo-Morlans M, Medina-Bolívar OC, Hernández-Acosta NC, González-Gómez M, et al. (2009)."Neurons in the white matter of the adult human neocortex".Frontiers in Neuroanatomy.3:7.doi:10.3389/neuro.05.007.2009.PMC2697018.PMID19543540.
- ^Smalheiser NR, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, et al. (February 2000)."Expression of reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells".Proceedings of the National Academy of Sciences of the United States of America.97(3): 1281–6.Bibcode:2000PNAS...97.1281S.doi:10.1073/pnas.97.3.1281.PMC15597.PMID10655522.
- ^Samama B, Boehm N (July 2005)."Reelin immunoreactivity in lymphatics and liver during development and adult life".The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology.285(1): 595–9.doi:10.1002/ar.a.20202.PMID15912522.
- ^abKobold D, Grundmann A, Piscaglia F, Eisenbach C, Neubauer K, Steffgen J, et al. (May 2002). "Expression of reelin in hepatic stellate cells and during hepatic tissue repair: a novel marker for the differentiation of HSC from other liver myofibroblasts".Journal of Hepatology.36(5): 607–13.doi:10.1016/S0168-8278(02)00050-8.PMID11983443.
- ^abPulido JS, Sugaya I, Comstock J, Sugaya K (June 2007). "Reelin expression is upregulated following ocular tissue injury".Graefe's Archive for Clinical and Experimental Ophthalmology.245(6): 889–93.doi:10.1007/s00417-006-0458-4.PMID17120005.S2CID12397364.
- ^Buchaille R, Couble ML, Magloire H, Bleicher F (September 2000). "A substractive PCR-based cDNA library from human odontoblast cells: identification of novel genes expressed in tooth forming cells".Matrix Biology.19(5): 421–30.doi:10.1016/S0945-053X(00)00091-3.PMID10980418.
- ^Allard B, Magloire H, Couble ML, Maurin JC, Bleicher F (September 2006)."Voltage-gated sodium channels confer excitability to human odontoblasts: possible role in tooth pain transmission".The Journal of Biological Chemistry.281(39): 29002–10.doi:10.1074/jbc.M601020200.PMID16831873.
- ^Maurin JC, Couble ML, Didier-Bazes M, Brisson C, Magloire H, Bleicher F (August 2004). "Expression and localization of reelin in human odontoblasts".Matrix Biology.23(5): 277–85.doi:10.1016/j.matbio.2004.06.005.PMID15464360.
- ^PDB:2E26;Yasui N, Nogi T, Kitao T, Nakano Y, Hattori M, Takagi J (June 2007)."Structure of a receptor-binding fragment of reelin and mutational analysis reveal a recognition mechanism similar to endocytic receptors".Proceedings of the National Academy of Sciences of the United States of America.104(24): 9988–93.Bibcode:2007PNAS..104.9988Y.doi:10.1073/pnas.0700438104.PMC1891246.PMID17548821.
- ^abQuattrocchi CC, Wannenes F, Persico AM, Ciafré SA, D'Arcangelo G, Farace MG, et al. (January 2002)."Reelin is a serine protease of the extracellular matrix".The Journal of Biological Chemistry.277(1): 303–9.doi:10.1074/jbc.M106996200.hdl:11380/1250927.PMID11689558.
- ^abRoyaux I, Lambert de Rouvroit C, D'Arcangelo G, Demirov D, Goffinet AM (December 1997). "Genomic organization of the mouse reelin gene".Genomics.46(2): 240–50.doi:10.1006/geno.1997.4983.PMID9417911.
- ^PDB:2ddu;Nogi T, Yasui N, Hattori M, Iwasaki K, Takagi J (August 2006)."Structure of a signaling-competent reelin fragment revealed by X-ray crystallography and electron tomography".The EMBO Journal.25(15): 3675–83.doi:10.1038/sj.emboj.7601240.PMC1538547.PMID16858396.
- ^Nakano Y, Kohno T, Hibi T, Kohno S, Baba A, Mikoshiba K, et al. (July 2007)."The extremely conserved C-terminal region of Reelin is not necessary for secretion but is required for efficient activation of downstream signaling".The Journal of Biological Chemistry.282(28): 20544–52.doi:10.1074/jbc.M702300200.PMID17504759.
- ^Lambert de Rouvroit C, de Bergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet AM (March 1999). "Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase".Experimental Neurology.156(1): 214–7.doi:10.1006/exnr.1998.7007.PMID10192793.S2CID35222830.
- ^abcJossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM (January 2004)."The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development".The Journal of Neuroscience.24(2): 514–21.doi:10.1523/JNEUROSCI.3408-03.2004.PMC6730001.PMID14724251.
- ^abcJossin Y, Gui L, Goffinet AM (April 2007)."Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons".The Journal of Neuroscience.27(16): 4243–52.doi:10.1523/JNEUROSCI.0023-07.2007.PMC6672330.PMID17442808.
- ^abBlake SM, Strasser V, Andrade N, Duit S, Hofbauer R, Schneider WJ, et al. (November 2008)."Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration".The EMBO Journal.27(22): 3069–80.doi:10.1038/emboj.2008.223.PMC2585172.PMID18946489.
- ^Lennington JB, Yang Z, Conover JC (November 2003)."Neural stem cells and the regulation of adult neurogenesis".Reproductive Biology and Endocrinology.1:99.doi:10.1186/1477-7827-1-99.PMC293430.PMID14614786.
- ^abHartfuss E, Förster E, Bock HH, Hack MA, Leprince P, Luque JM, et al. (October 2003)."Reelin signaling directly affects radial glia morphology and biochemical maturation".Development.130(19): 4597–609.doi:10.1242/dev.00654.hdl:10261/333510.PMID12925587.
- ^abcdeNomura T, Takahashi M, Hara Y, Osumi N (January 2008). Reh T (ed.)."Patterns of neurogenesis and amplitude of Reelin expression are essential for making a mammalian-type cortex".PLOS ONE.3(1): e1454.Bibcode:2008PLoSO...3.1454N.doi:10.1371/journal.pone.0001454.PMC2175532.PMID18197264.
- ^Del Río JA, Heimrich B, Borrell V, Förster E, Drakew A, Alcántara S, et al. (January 1997). "A role for Cajal-Retzius cells and reelin in the development of hippocampal connections".Nature.385(6611): 70–4.Bibcode:1997Natur.385...70D.doi:10.1038/385070a0.PMID8985248.S2CID4352996.
- ^Borrell V, Del Río JA, Alcántara S, Derer M, Martínez A, D'Arcangelo G, et al. (February 1999)."Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections".The Journal of Neuroscience.19(4): 1345–58.doi:10.1523/JNEUROSCI.19-04-01345.1999.PMC6786030.PMID9952412.
- ^abHack I, Bancila M, Loulier K, Carroll P, Cremer H (October 2002). "Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis".Nature Neuroscience.5(10): 939–45.doi:10.1038/nn923.PMID12244323.S2CID7096018.
- ^abcYoshida M, Assimacopoulos S, Jones KR, Grove EA (February 2006). "Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order".Development.133(3): 537–45.doi:10.1242/dev.02209.PMID16410414.S2CID1702450.
- ^Yip YP, Mehta N, Magdaleno S, Curran T, Yip JW (July 2009). "Ectopic expression of reelin alters migration of sympathetic preganglionic neurons in the spinal cord".The Journal of Comparative Neurology.515(2): 260–8.doi:10.1002/cne.22044.PMID19412957.S2CID21832778.
- ^abCampo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P (2009). Okazawa H (ed.)."Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis".PLOS ONE.4(5): e5505.Bibcode:2009PLoSO...4.5505C.doi:10.1371/journal.pone.0005505.PMC2675077.PMID19430527.
- ^INSERM – Olivier Manzoni – Physiopathology of Synaptic Transmission and PlasticityArchived25 November 2006 at theWayback Machine– Bordo neuroscience institute.
- ^Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, et al. (June 2005)."Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro".The Journal of Neuroscience.25(26): 6127–36.doi:10.1523/JNEUROSCI.1757-05.2005.PMC6725049.PMID15987942.
- ^abGroc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P (September 2007)."NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin".The Journal of Neuroscience.27(38): 10165–75.doi:10.1523/JNEUROSCI.1772-07.2007.PMC6672660.PMID17881522.
- ^Liu XB, Murray KD, Jones EG (October 2004)."Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development".The Journal of Neuroscience.24(40): 8885–95.doi:10.1523/JNEUROSCI.2476-04.2004.PMC6729956.PMID15470155.
- ^Andrade N, Komnenovic V, Blake SM, Jossin Y, Howell B, Goffinet A, et al. (May 2007)."ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin".Proceedings of the National Academy of Sciences of the United States of America.104(20): 8508–13.Bibcode:2007PNAS..104.8508A.doi:10.1073/pnas.0611391104.PMC1895980.PMID17494763.
- ^Frotscher M, Haas CA, Förster E (June 2003)."Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold".Cerebral Cortex.13(6): 634–40.doi:10.1093/cercor/13.6.634.PMID12764039.
- ^Bar I, Lambert de Rouvroit C, Goffinet AM (December 2000). "The evolution of cortical development. An hypothesis based on the role of the Reelin signaling pathway".Trends in Neurosciences.23(12): 633–8.doi:10.1016/S0166-2236(00)01675-1.PMID11137154.S2CID13568642.
- ^Molnár Z, Métin C, Stoykova A, Tarabykin V, Price DJ, Francis F, et al. (February 2006)."Comparative aspects of cerebral cortical development".The European Journal of Neuroscience.23(4): 921–34.doi:10.1111/j.1460-9568.2006.04611.x.PMC1931431.PMID16519657.
- ^abPérez-García CG, González-Delgado FJ, Suárez-Solá ML, Castro-Fuentes R, Martín-Trujillo JM, Ferres-Torres R, et al. (January 2001). "Reelin-immunoreactive neurons in the adult vertebrate pallium".Journal of Chemical Neuroanatomy.21(1): 41–51.doi:10.1016/S0891-0618(00)00104-6.PMID11173219.S2CID23395046.
- ^Costagli A, Kapsimali M, Wilson SW, Mione M (August 2002). "Conserved and divergent patterns of Reelin expression in the zebrafish central nervous system".The Journal of Comparative Neurology.450(1): 73–93.doi:10.1002/cne.10292.PMID12124768.S2CID23110916.
- ^Goffinet AM (November 2006)."What makes us human? A biased view from the perspective of comparative embryology and mouse genetics".Journal of Biomedical Discovery and Collaboration.1:16.doi:10.1186/1747-5333-1-16.PMC1769396.PMID17132178.
- ^Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, Pedersen JS, et al. (September 2006)."An RNA gene expressed during cortical development evolved rapidly in humans"(PDF).Nature.443(7108): 167–72.Bibcode:2006Natur.443..167P.doi:10.1038/nature05113.PMID16915236.S2CID18107797.
- ^Williamson SH, Hubisz MJ, Clark AG, Payseur BA, Bustamante CD, Nielsen R (June 2007)."Localizing recent adaptive evolution in the human genome".PLOS Genetics.3(6): e90.doi:10.1371/journal.pgen.0030090.PMC1885279.PMID17542651.
- ^Wade N (26 June 2007)."Humans Have Spread Globally, and Evolved Locally".The New York Times.Retrieved23 August2008.
- ^abZhang G, Assadi AH, McNeil RS, Beffert U, Wynshaw-Boris A, Herz J, et al. (February 2007). Mueller U (ed.)."The Pafah1b complex interacts with the reelin receptor VLDLR".PLOS ONE.2(2): e252.Bibcode:2007PLoSO...2..252Z.doi:10.1371/journal.pone.0000252.PMC1800349.PMID17330141.
- ^D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T (October 1999)."Reelin is a ligand for lipoprotein receptors".Neuron.24(2): 471–9.doi:10.1016/S0896-6273(00)80860-0.PMID10571240.S2CID14631418.
- ^Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, et al. (October 1999)."Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation".Neuron.24(2): 481–9.doi:10.1016/S0896-6273(00)80861-2.PMID10571241.S2CID243043.
- ^Andersen OM, Benhayon D, Curran T, Willnow TE (August 2003). "Differential binding of ligands to the apolipoprotein E receptor 2".Biochemistry.42(31): 9355–64.doi:10.1021/bi034475p.PMID12899622.
- ^Benhayon D, Magdaleno S, Curran T (April 2003). "Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1".Brain Research. Molecular Brain Research.112(1–2): 33–45.doi:10.1016/S0169-328X(03)00032-9.PMID12670700.
- ^Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, et al. (November 2007)."Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons".Development.134(21): 3883–91.doi:10.1242/dev.005447.PMID17913789.
- ^Schmid RS, Jo R, Shelton S, Kreidberg JA, Anton ES (October 2005)."Reelin, integrin and DAB1 interactions during embryonic cerebral cortical development".Cerebral Cortex.15(10): 1632–6.doi:10.1093/cercor/bhi041.PMID15703255.
- ^Senzaki K, Ogawa M, Yagi T (December 1999)."Proteins of the CNR family are multiple receptors for Reelin".Cell.99(6): 635–47.doi:10.1016/S0092-8674(00)81552-4.PMID10612399.S2CID14277878.
- ^Hibi T, Hattori M (April 2009)."The N-terminal fragment of Reelin is generated after endocytosis and released through the pathway regulated by Rab11".FEBS Letters.583(8): 1299–303.Bibcode:2009FEBSL.583.1299H.doi:10.1016/j.febslet.2009.03.024.PMID19303411.S2CID43542615.
- ^Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA (April 2009)."The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons".Proceedings of the National Academy of Sciences of the United States of America.106(17): 7227–32.Bibcode:2009PNAS..106.7227C.doi:10.1073/pnas.0810764106.PMC2678467.PMID19366679.
- ^Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Müller U (December 2007)."Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex".The Journal of Neuroscience.27(50): 13854–65.doi:10.1523/JNEUROSCI.4494-07.2007.PMC6673609.PMID18077697.
- ^Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, et al. (August 2005)."Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2".Neuron.47(4): 567–79.doi:10.1016/j.neuron.2005.07.007.PMID16102539.S2CID5854936.
- ^Miller CA, Sweatt JD (March 2007)."Covalent modification of DNA regulates memory formation".Neuron.53(6): 857–69.doi:10.1016/j.neuron.2007.02.022.PMID17359920.S2CID62791264.
- ^abMatsuki T, Pramatarova A, Howell BW (June 2008)."Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis".Journal of Cell Science.121(11): 1869–75.doi:10.1242/jcs.027334.PMC2430739.PMID18477607.
- ^Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA (April 2004)."Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons".Current Biology.14(7): 606–10.Bibcode:2004CBio...14..606B.doi:10.1016/j.cub.2004.03.038.PMID15062102.S2CID52887334.
- ^Park TJ, Curran T (December 2008)."Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway".The Journal of Neuroscience.28(50): 13551–62.doi:10.1523/JNEUROSCI.4323-08.2008.PMC2628718.PMID19074029.
- ^abKeilani S, Sugaya K (July 2008)."Reelin induces a radial glial phenotype in human neural progenitor cells by activation of Notch-1".BMC Developmental Biology.8(1): 69.doi:10.1186/1471-213X-8-69.PMC2447831.PMID18593473.
- ^Lugli G, Krueger JM, Davis JM, Persico AM, Keller F, Smalheiser NR (September 2003)."Methodological factors influencing measurement and processing of plasma reelin in humans".BMC Biochemistry.4:9.doi:10.1186/1471-2091-4-9.PMC200967.PMID12959647.
- ^Howell BW, Gertler FB, Cooper JA (January 1997)."Mouse disabled (mDab1): a Src binding protein implicated in neuronal development".The EMBO Journal.16(1): 121–32.doi:10.1093/emboj/16.1.121.PMC1169619.PMID9009273.
- ^Arnaud L, Ballif BA, Förster E, Cooper JA (January 2003)."Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development".Current Biology.13(1): 9–17.Bibcode:2003CBio...13....9A.doi:10.1016/S0960-9822(02)01397-0.PMID12526739.S2CID1739505.
- ^Feng L, Allen NS, Simo S, Cooper JA (November 2007)."Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development".Genes & Development.21(21): 2717–30.doi:10.1101/gad.1604207.PMC2045127.PMID17974915.
- ^Kerjan G, Gleeson JG (November 2007)."A missed exit: Reelin sets in motion Dab1 polyubiquitination to put the break on neuronal migration".Genes & Development.21(22): 2850–4.doi:10.1101/gad.1622907.PMID18006681.
- ^Utsunomiya-Tate N, Kubo K, Tate S, Kainosho M, Katayama E, Nakajima K, et al. (August 2000)."Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody".Proceedings of the National Academy of Sciences of the United States of America.97(17): 9729–34.Bibcode:2000PNAS...97.9729U.doi:10.1073/pnas.160272497.PMC16933.PMID10920200.
- ^Kubo K, Mikoshiba K, Nakajima K (August 2002). "Secreted Reelin molecules form homodimers".Neuroscience Research.43(4): 381–8.doi:10.1016/S0168-0102(02)00068-8.PMID12135781.S2CID10656560.
- ^abStrasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, et al. (February 2004)."Receptor clustering is involved in Reelin signaling".Molecular and Cellular Biology.24(3): 1378–86.doi:10.1128/MCB.24.3.1378-1386.2004.PMC321426.PMID14729980.
- ^Chai X, Förster E, Zhao S, Bock HH, Frotscher M (January 2009)."Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3".The Journal of Neuroscience.29(1): 288–99.doi:10.1523/JNEUROSCI.2934-08.2009.PMC6664910.PMID19129405.
- ^Frotscher M, Chai X, Bock HH, Haas CA, Förster E, Zhao S (November 2009). "Role of Reelin in the development and maintenance of cortical lamination".Journal of Neural Transmission.116(11): 1451–5.doi:10.1007/s00702-009-0228-7.PMID19396394.S2CID1310387.
- ^Arnaud L, Ballif BA, Cooper JA (December 2003)."Regulation of protein tyrosine kinase signaling by substrate degradation during brain development".Molecular and Cellular Biology.23(24): 9293–302.doi:10.1128/MCB.23.24.9293-9302.2003.PMC309695.PMID14645539.
- ^Ohshima T, Suzuki H, Morimura T, Ogawa M, Mikoshiba K (April 2007). "Modulation of Reelin signaling by Cyclin-dependent kinase 5".Brain Research.1140:84–95.doi:10.1016/j.brainres.2006.01.121.PMID16529723.S2CID23991327.
- ^Keshvara L, Magdaleno S, Benhayon D, Curran T (June 2002)."Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling".The Journal of Neuroscience.22(12): 4869–77.doi:10.1523/JNEUROSCI.22-12-04869.2002.PMC6757745.PMID12077184.
- ^Kobayashi S, Ishiguro K, Omori A, Takamatsu M, Arioka M, Imahori K, et al. (December 1993). "A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30 kDa subunit of tau protein kinase II, a proline-directed protein kinase associated with microtubule".FEBS Letters.335(2): 171–5.Bibcode:1993FEBSL.335..171K.doi:10.1016/0014-5793(93)80723-8.PMID8253190.S2CID26474408.
- ^Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J (December 2002)."Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta".The Journal of Biological Chemistry.277(51): 49958–64.doi:10.1074/jbc.M209205200.PMID12376533.
- ^Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, et al. (December 2000)."A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system".Neuron.28(3): 681–96.doi:10.1016/S0896-6273(00)00146-X.PMID11163259.S2CID17738599.
- ^abBeffert U, Weeber EJ, Morfini G, Ko J, Brady ST, Tsai LH, et al. (February 2004)."Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission".The Journal of Neuroscience.24(8): 1897–906.doi:10.1523/JNEUROSCI.4084-03.2004.PMC6730409.PMID14985430.
- ^Ohshima T, Ogawa M, Hirasawa M, Longenecker G, Ishiguro K, Pant HC, et al. (February 2001)."Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain".Proceedings of the National Academy of Sciences of the United States of America.98(5): 2764–9.Bibcode:2001PNAS...98.2764O.doi:10.1073/pnas.051628498.PMC30213.PMID11226314.
- ^Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, et al. (September 2000). "Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations".Nature Genetics.26(1): 93–6.doi:10.1038/79246.PMID10973257.S2CID67748801.
- ^Crino P (November 2001)."New RELN Mutation Associated with Lissencephaly and Epilepsy".Epilepsy Currents.1(2): 72–73.doi:10.1046/j.1535-7597.2001.00017.x.PMC320825.PMID15309195.
- ^Zaki M, Shehab M, El-Aleem AA, Abdel-Salam G, Koeller HB, Ilkin Y, et al. (May 2007). "Identification of a novel recessive RELN mutation using a homozygous balanced reciprocal translocation".American Journal of Medical Genetics. Part A.143A(9): 939–44.doi:10.1002/ajmg.a.31667.PMID17431900.S2CID19126812.
- ^Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. (December 1998)."A decrease of reelin expression as a putative vulnerability factor in schizophrenia".Proceedings of the National Academy of Sciences of the United States of America.95(26): 15718–23.Bibcode:1998PNAS...9515718I.doi:10.1073/pnas.95.26.15718.PMC28110.PMID9861036.
- ^abGuidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. (November 2000). "Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study".Archives of General Psychiatry.57(11): 1061–9.doi:10.1001/archpsyc.57.11.1061.PMID11074872.
- ^abFatemi SH, Hossein Fatemi S, Stary JM, Earle JA, Araghi-Niknam M, Eagan E (January 2005). "GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum".Schizophrenia Research.72(2–3): 109–22.doi:10.1016/j.schres.2004.02.017.PMID15560956.S2CID35193802.
- ^abVeldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, et al. (March 2007)."Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder".Schizophrenia Research.91(1–3): 51–61.doi:10.1016/j.schres.2006.11.029.PMC1876737.PMID17270400.
- ^Eastwood SL, Harrison PJ (September 2003). "Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis".Molecular Psychiatry.8(9): 769, 821–31.doi:10.1038/sj.mp.4001371.PMID12931209.S2CID25020557.
- ^Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, et al. (April 2005). "Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report".American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics.134B(1): 60–6.doi:10.1002/ajmg.b.30140.PMID15717292.S2CID23169492.
- ^Fatemi SH, Kroll JL, Stary JM (October 2001). "Altered levels of Reelin and its isoforms in schizophrenia and mood disorders".NeuroReport.12(15): 3209–15.doi:10.1097/00001756-200110290-00014.PMID11711858.S2CID43077109.
- ^Knable MB, Torrey EF, Webster MJ, Bartko JJ (July 2001). "Multivariate analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium".Brain Research Bulletin.55(5): 651–9.doi:10.1016/S0361-9230(01)00521-4.PMID11576762.S2CID23427111.
- ^Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, et al. (June 2005)."Reelin promoter hypermethylation in schizophrenia".Proceedings of the National Academy of Sciences of the United States of America.102(26): 9341–6.Bibcode:2005PNAS..102.9341G.doi:10.1073/pnas.0503736102.PMC1166626.PMID15961543.
- ^Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A (August 2005)."Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia".Proceedings of the National Academy of Sciences of the United States of America.102(35): 12578–83.Bibcode:2005PNAS..10212578D.doi:10.1073/pnas.0505394102.PMC1194936.PMID16113080.
- ^Pollin W, Cardon PV, Kety SS (January 1961). "Effects of amino acid feedings in schizophrenic patients treated with iproniazid".Science.133(3446): 104–5.Bibcode:1961Sci...133..104P.doi:10.1126/science.133.3446.104.PMID13736870.S2CID32080078.
- ^Brune GG, Himwich HE (May 1962). "Effects of methionine loading on the behavior of schizophrenic patients".The Journal of Nervous and Mental Disease.134(5): 447–50.doi:10.1097/00005053-196205000-00007.PMID13873983.S2CID46617457.
- ^Park LC, Baldessarini RJ, Kety SS (April 1965). "Methionine Effects on Chronic Schizophrenics".Archives of General Psychiatry.12(4): 346–51.doi:10.1001/archpsyc.1965.01720340018003.PMID14258360.
- ^Antun FT, Burnett GB, Cooper AJ, Daly RJ, Smythies JR, Zealley AK (June 1971). "The effects of L-methionine (without MAOI) in schizophrenia".Journal of Psychiatric Research.8(2): 63–71.doi:10.1016/0022-3956(71)90009-4.PMID4932991.
- ^Grayson DR, Chen Y, Dong E, Kundakovic M, Guidotti A (April 2009)."From trans-methylation to cytosine methylation: evolution of the methylation hypothesis of schizophrenia".Epigenetics.4(3): 144–9.doi:10.4161/epi.4.3.8534.PMID19395859.
- ^Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A (April 2007). "Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection".Molecular Psychiatry.12(4): 385–97.doi:10.1038/sj.mp.4001954.PMID17264840.S2CID24045153.
- ^Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, et al. (January 2004)."DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains".Proceedings of the National Academy of Sciences of the United States of America.101(1): 348–53.Bibcode:2004PNAS..101..348V.doi:10.1073/pnas.2637013100.PMC314188.PMID14684836.
- ^Veldic M, Guidotti A, Maloku E, Davis JM, Costa E (February 2005)."In psychosis, cortical interneurons overexpress DNA-methyltransferase 1".Proceedings of the National Academy of Sciences of the United States of America.102(6): 2152–7.Bibcode:2005PNAS..102.2152V.doi:10.1073/pnas.0409665102.PMC548582.PMID15684088.
- ^Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, et al. (March 2005). "Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice".Biological Psychiatry.57(5): 500–9.doi:10.1016/j.biopsych.2004.11.046.PMID15737665.S2CID29868395.
- ^Chen Y, Sharma RP, Costa RH, Costa E, Grayson DR (July 2002)."On the epigenetic regulation of the human reelin promoter".Nucleic Acids Research.30(13): 2930–9.doi:10.1093/nar/gkf401.PMC117056.PMID12087179.
- ^Mitchell CP, Chen Y, Kundakovic M, Costa E, Grayson DR (April 2005). "Histone deacetylase inhibitors decrease reelin promoter methylation in vitro".Journal of Neurochemistry.93(2): 483–92.doi:10.1111/j.1471-4159.2005.03040.x.PMID15816871.S2CID12445076.
- ^Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, et al. (December 2002)."An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability".Proceedings of the National Academy of Sciences of the United States of America.99(26): 17095–100.Bibcode:2002PNAS...9917095T.doi:10.1073/pnas.262658999.PMC139275.PMID12481028.
- ^Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M (June 2007)."Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars".Proceedings of the National Academy of Sciences of the United States of America.104(24): 10164–9.Bibcode:2007PNAS..10410164B.doi:10.1073/pnas.0703806104.PMC1888575.PMID17553960.
- ^Dong E, Guidotti A, Grayson DR, Costa E (March 2007)."Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters".Proceedings of the National Academy of Sciences of the United States of America.104(11): 4676–81.Bibcode:2007PNAS..104.4676D.doi:10.1073/pnas.0700529104.PMC1815468.PMID17360583.
- ^Kundakovic M, Chen Y, Costa E, Grayson DR (March 2007). "DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes".Molecular Pharmacology.71(3): 644–53.doi:10.1124/mol.106.030635.PMID17065238.S2CID18421124.
- ^Kundakovic M, Chen Y, Guidotti A, Grayson DR (February 2009)."The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes".Molecular Pharmacology.75(2): 342–54.doi:10.1124/mol.108.051763.PMC2684898.PMID19029285.
- ^Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, et al. (January 2007). "S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis".NeuroReport.18(1): 57–60.doi:10.1097/WNR.0b013e32800fefd7.PMID17259861.S2CID25378736.
- ^abWedenoja J, Loukola A, Tuulio-Henriksson A, Paunio T, Ekelund J, Silander K, et al. (July 2008). "Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families".Molecular Psychiatry.13(7): 673–84.doi:10.1038/sj.mp.4002047.PMID17684500.S2CID20658493.
- ^Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, et al. (February 2008)."Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women".PLOS Genetics.4(2): e28.doi:10.1371/journal.pgen.0040028.PMC2242812.PMID18282107.free full text
- ^Wedenoja J, Tuulio-Henriksson A, Suvisaari J, Loukola A, Paunio T, Partonen T, et al. (May 2010)."Replication of association between working memory and Reelin, a potential modifier gene in schizophrenia".Biological Psychiatry.67(10): 983–91.doi:10.1016/j.biopsych.2009.09.026.PMC3083525.PMID19922905.
- ^Gregório SP, Sallet PC, Do KA, Lin E, Gattaz WF, Dias-Neto E (January 2009). "Polymorphisms in genes involved in neurodevelopment may be associated with altered brain morphology in schizophrenia: preliminary evidence".Psychiatry Research.165(1–2): 1–9.doi:10.1016/j.psychres.2007.08.011.PMID19054571.S2CID43548414.
- ^abSuzuki K, Nakamura K, Iwata Y, Sekine Y, Kawai M, Sugihara G, et al. (January 2008). "Decreased expression of reelin receptor VLDLR in peripheral lymphocytes of drug-naive schizophrenic patients".Schizophrenia Research.98(1–3): 148–56.doi:10.1016/j.schres.2007.09.029.PMID17936586.S2CID45594329.
- ^Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA (January 2009)."Reduced dendritic spine density in auditory cortex of subjects with schizophrenia".Neuropsychopharmacology.34(2): 374–89.doi:10.1038/npp.2008.67.PMC2775717.PMID18463626.
- ^Glantz LA, Lewis DA (January 2000). "Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia".Archives of General Psychiatry.57(1): 65–73.doi:10.1001/archpsyc.57.1.65.PMID10632234.
- ^Rodriguez MA, Pesold C, Liu WS, Kriho V, Guidotti A, Pappas GD, et al. (March 2000)."Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex".Proceedings of the National Academy of Sciences of the United States of America.97(7): 3550–5.Bibcode:2000PNAS...97.3550R.doi:10.1073/pnas.050589797.PMC16277.PMID10725376.
- ^Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C (October 2001)."Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability".Neurobiology of Disease.8(5): 723–42.doi:10.1006/nbdi.2001.0436.PMID11592844.S2CID31279244.
- ^Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW (May 2003)."Disruption of the neuronal PAS3 gene in a family affected with schizophrenia".Journal of Medical Genetics.40(5): 325–32.doi:10.1136/jmg.40.5.325.PMC1735455.PMID12746393.
- ^Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, Han T, et al. (September 2004)."Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors".Proceedings of the National Academy of Sciences of the United States of America.101(37): 13648–53.Bibcode:2004PNAS..10113648E.doi:10.1073/pnas.0405310101.PMC518807.PMID15347806.
- ^Chen Z, Schwahn BC, Wu Q, He X, Rozen R (August 2005). "Postnatal cerebellar defects in mice deficient in methylenetetrahydrofolate reductase".International Journal of Developmental Neuroscience.23(5): 465–74.doi:10.1016/j.ijdevneu.2005.05.007.PMID15979267.S2CID37922852.
- ^Gene Overview of All Published Schizophrenia-Association Studies for GRIN2BArchived13 September 2010 at theWayback Machine– Schizophrenia Gene Database.
- ^abWang GS, Hong CJ, Yen TY, Huang HY, Ou Y, Huang TN, et al. (April 2004)."Transcriptional modification by a CASK-interacting nucleosome assembly protein".Neuron.42(1): 113–28.doi:10.1016/S0896-6273(04)00139-4.PMID15066269.S2CID14383387.
- ^Podhorna J, Didriksen M (August 2004)."The heterozygous reeler mouse: behavioural phenotype".Behavioural Brain Research.153(1): 43–54.doi:10.1016/j.bbr.2003.10.033.PMID15219705.S2CID538066.
- ^Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jones EG (May 1996). "Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients".Archives of General Psychiatry.53(5): 425–36.doi:10.1001/archpsyc.1996.01830050061010.PMID8624186.
- ^Joshi D, Fung SJ, Rothwell A, Weickert CS (November 2012). "Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia".Biological Psychiatry.72(9): 725–33.doi:10.1016/j.biopsych.2012.06.021.PMID22841514.S2CID8400626.
- ^abcMatigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, et al. (2010)."Disease-specific, neurosphere-derived cells as models for brain disorders"(PDF).Disease Models & Mechanisms.3(11–12): 785–98.doi:10.1242/dmm.005447.PMID20699480.
- ^Topol A, Zhu S, Hartley BJ, English J, Hauberg ME, Tran N, et al. (May 2016)."Dysregulation of miRNA-9 in a Subset of Schizophrenia Patient-Derived Neural Progenitor Cells".Cell Reports.15(5): 1024–1036.doi:10.1016/j.celrep.2016.03.090.PMC4856588.PMID27117414.
- ^abcdTee JY, Sutharsan R, Fan Y, Mackay-Sim A (2016)."Schizophrenia patient-derived olfactory neurosphere-derived cells do not respond to extracellular reelin".npj Schizophrenia.2:16027.doi:10.1038/npjschz.2016.27.PMC4994154.PMID27602387.
- ^Goes FS, Willour VL, Zandi PP, Belmonte PL, MacKinnon DF, Mondimore FM, et al. (March 2010)."Sex-specific association of the Reelin gene with bipolar disorder".American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics.153B(2): 549–553.doi:10.1002/ajmg.b.31018.PMC3032172.PMID19691043.
- ^Lammert DB, Howell BW (31 March 2016)."RELN Mutations in Autism Spectrum Disorder".Frontiers in Cellular Neuroscience.10(84): 84.doi:10.3389/fncel.2016.00084.PMC4814460.PMID27064498.
- ^Persico AM, D'Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, et al. (March 2001). "Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder".Molecular Psychiatry.6(2): 150–9.doi:10.1038/sj.mp.4000850.PMID11317216.S2CID1472363.
- ^Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, et al. (2002). "Reelin gene alleles and susceptibility to autism spectrum disorders".Molecular Psychiatry.7(9): 1012–7.doi:10.1038/sj.mp.4001124.PMID12399956.S2CID30799924.
- ^Bonora E, Beyer KS, Lamb JA, Parr JR, Klauck SM, Benner A, et al. (October 2003). "Analysis of reelin as a candidate gene for autism".Molecular Psychiatry.8(10): 885–92.doi:10.1038/sj.mp.4001310.PMID14515139.S2CID32279857.
- ^Devlin B, Bennett P, Dawson G, Figlewicz DA, Grigorenko EL, McMahon W, et al. (April 2004)."Alleles of a reelin CGG repeat do not convey liability to autism in a sample from the CPEA network"(PDF).American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics.126B(1): 46–50.doi:10.1002/ajmg.b.20125.PMID15048647.S2CID14966506.
- ^Holt R, Barnby G, Maestrini E, Bacchelli E, Brocklebank D, Sousa I, et al. (September 2010)."Linkage and candidate gene studies of autism spectrum disorders in European populations".European Journal of Human Genetics.18(9): 1013–9.doi:10.1038/ejhg.2010.69.PMC2987412.PMID20442744.
- ^Pardo CA, Eberhart CG (October 2007)."The neurobiology of autism".Brain Pathology.17(4): 434–47.doi:10.1111/j.1750-3639.2007.00102.x.PMC8095519.PMID17919129.S2CID37048272.
- ^Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, et al. (July 2002)."Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy".The Journal of Neuroscience.22(14): 5797–802.doi:10.1523/JNEUROSCI.22-14-05797.2002.PMC6757930.PMID12122039.
- ^Heinrich C, Nitta N, Flubacher A, Müller M, Fahrner A, Kirsch M, et al. (April 2006)."Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus".The Journal of Neuroscience.26(17): 4701–13.doi:10.1523/JNEUROSCI.5516-05.2006.PMC6674063.PMID16641251.
- ^Kobow K, Jeske I, Hildebrandt M, Hauke J, Hahnen E, Buslei R, et al. (April 2009)."Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy".Journal of Neuropathology and Experimental Neurology.68(4): 356–64.doi:10.1097/NEN.0b013e31819ba737.PMID19287316.
- ^Gong C, Wang TW, Huang HS, Parent JM (February 2007)."Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus".The Journal of Neuroscience.27(8): 1803–11.doi:10.1523/JNEUROSCI.3111-06.2007.PMC6673551.PMID17314278.
- ^Müller MC, Osswald M, Tinnes S, Häussler U, Jacobi A, Förster E, et al. (April 2009). "Exogenous reelin prevents granule cell dispersion in experimental epilepsy".Experimental Neurology.216(2): 390–7.doi:10.1016/j.expneurol.2008.12.029.PMID19185570.S2CID17173306.
- ^abHerz J, Beffert U (October 2000). "Apolipoprotein E receptors: linking brain development and Alzheimer's disease".Nature Reviews. Neuroscience.1(1): 51–8.doi:10.1038/35036221.PMID11252768.S2CID27105032.
- ^Herz J, Chen Y (November 2006). "Reelin, lipoprotein receptors and synaptic plasticity".Nature Reviews. Neuroscience.7(11): 850–9.doi:10.1038/nrn2009.PMID17053810.S2CID44317115.
- ^Botella-López A, Burgaya F, Gavín R, García-Ayllón MS, Gómez-Tortosa E, Peña-Casanova J, et al. (April 2006)."Reelin expression and glycosylation patterns are altered in Alzheimer's disease".Proceedings of the National Academy of Sciences of the United States of America.103(14): 5573–8.Bibcode:2006PNAS..103.5573B.doi:10.1073/pnas.0601279103.PMC1414634.PMID16567613.
- ^Wirths O, Multhaup G, Czech C, Blanchard V, Tremp G, Pradier L, et al. (December 2001). "Reelin in plaques of beta-amyloid precursor protein and presenilin-1 double-transgenic mice".Neuroscience Letters.316(3): 145–8.doi:10.1016/S0304-3940(01)02399-0.PMID11744223.S2CID35475092.
- ^Seripa D, Matera MG, Franceschi M, Daniele A, Bizzarro A, Rinaldi M, et al. (July 2008). "The RELN locus in Alzheimer's disease".Journal of Alzheimer's Disease.14(3): 335–44.doi:10.3233/jad-2008-14308.PMID18599960.
- ^Baloyannis SJ (July 2005). "Morphological and morphometric alterations of Cajal-Retzius cells in early cases of Alzheimer's disease: a Golgi and electron microscope study".The International Journal of Neuroscience.115(7): 965–80.doi:10.1080/00207450590901396.PMID16051543.S2CID36197073.
- ^Baloyannis SJ, Costa V, Mauroudis I, Psaroulis D, Manolides SL, Manolides LS (April 2007). "Dendritic and spinal pathology in the acoustic cortex in Alzheimer's disease: morphological and morphometric estimation by Golgi technique and electron microscopy".Acta Oto-Laryngologica.127(4): 351–4.doi:10.1080/00016480601126986.PMID17453452.S2CID21625263.
- ^Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, et al. (June 2009)."Interaction of reelin with amyloid precursor protein promotes neurite outgrowth".The Journal of Neuroscience.29(23): 7459–73.doi:10.1523/JNEUROSCI.4872-08.2009.PMC2759694.PMID19515914.
- ^Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J (September 2009)."Reelin signaling antagonizes beta-amyloid at the synapse".Proceedings of the National Academy of Sciences of the United States of America.106(37): 15938–43.Bibcode:2009PNAS..10615938D.doi:10.1073/pnas.0908176106.PMC2747222.PMID19805234.
- ^Chen Y, Durakoglugil MS, Xian X, Herz J (June 2010)."ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling".Proceedings of the National Academy of Sciences of the United States of America.107(26): 12011–6.Bibcode:2010PNAS..10712011C.doi:10.1073/pnas.0914984107.PMC2900641.PMID20547867.
- ^Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M (February 2006)."Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers".Gastroenterology.130(2): 548–65.doi:10.1053/j.gastro.2005.11.008.PMID16472607.
- ^Perrone G, Vincenzi B, Zagami M, Santini D, Panteri R, Flammia G, et al. (March 2007)."Reelin expression in human prostate cancer: a marker of tumor aggressiveness based on correlation with grade".Modern Pathology.20(3): 344–51.doi:10.1038/modpathol.3800743.PMID17277764.
- ^Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F (June 2007)."Human embryonic and neuronal stem cell markers in retinoblastoma".Molecular Vision.13:823–32.PMC2768758.PMID17615543.
- ^Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, et al. (January 2012)."The genetic basis of early T-cell precursor acute lymphoblastic leukaemia".Nature.481(7380): 157–63.Bibcode:2012Natur.481..157Z.doi:10.1038/nature10725.PMC3267575.PMID22237106.
- ^Schrauwen I, Ealy M, Huentelman MJ, Thys M, Homer N, Vanderstraeten K, et al. (March 2009)."A genome-wide analysis identifies genetic variants in the RELN gene associated with otosclerosis".American Journal of Human Genetics.84(3): 328–38.doi:10.1016/j.ajhg.2009.01.023.PMC2667982.PMID19230858.
- ^Delahaye NF, Coltel N, Puthier D, Barbier M, Benech P, Joly F, et al. (December 2007)."Gene expression analysis reveals early changes in several molecular pathways in cerebral malaria-susceptible mice versus cerebral malaria-resistant mice".BMC Genomics.8:452.doi:10.1186/1471-2164-8-452.PMC2246131.PMID18062806.
- ^Garshasbi M, Mahmoudi M, Razmara E, Vojdanian M, Aslani S, Farhadi E, et al. (June 2020)."Identification of RELN variant p.(Ser2486Gly) in an Iranian family with ankylosing spondylitis; the first association of RELN and AS".European Journal of Human Genetics.28(6): 754–762.doi:10.1038/s41431-020-0573-4.PMC7253431.PMID32001840.
- ^"'Reelin' in a New Treatment for Multiple Sclerosis ".12 August 2020.
- ^abSmit-Rigter LA, Champagne DL, van Hooft JA (2009). Linden R (ed.)."Lifelong impact of variations in maternal care on dendritic structure and function of cortical layer 2/3 pyramidal neurons in rat offspring".PLOS ONE.4(4): e5167.Bibcode:2009PLoSO...4.5167S.doi:10.1371/journal.pone.0005167.PMC2663818.PMID19357777.
- ^Weaver IC, Meaney MJ, Szyf M (February 2006)."Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood".Proceedings of the National Academy of Sciences of the United States of America.103(9): 3480–5.Bibcode:2006PNAS..103.3480W.doi:10.1073/pnas.0507526103.PMC1413873.PMID16484373.
- ^Lussier AL, Caruncho HJ, Kalynchuk LE (August 2009). "Repeated exposure to corticosterone, but not restraint, decreases the number of reelin-positive cells in the adult rat hippocampus".Neuroscience Letters.460(2): 170–4.doi:10.1016/j.neulet.2009.05.050.PMID19477232.S2CID5305922.
- ^Lintas C, Persico AM (January 2010). "Neocortical RELN promoter methylation increases significantly after puberty".NeuroReport.21(2): 114–8.doi:10.1097/WNR.0b013e328334b343.PMID19952965.S2CID206137259.
- ^Ishii K, Kubo KI, Nakajima K (18 October 2016)."Reelin and Neuropsychiatric Disorders".Frontiers in Cellular Neuroscience.10:229.doi:10.3389/fncel.2016.00229.PMC5067484.PMID27803648.
- ^Dong E, Nelson M, Grayson DR, Costa E, Guidotti A (September 2008)."Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation".Proceedings of the National Academy of Sciences of the United States of America.105(36): 13614–9.Bibcode:2008PNAS..10513614D.doi:10.1073/pnas.0805493105.PMC2533238.PMID18757738.
- ^Fatemi SH, Reutiman TJ, Folsom TD (June 2009). "Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats".Schizophrenia Research.111(1–3): 138–52.doi:10.1016/j.schres.2009.03.002.PMID19359144.S2CID37048872.
Further reading
[edit]- Fatemi SH (March 2005). "Reelin glycoprotein: structure, biology and roles in health and disease".Molecular Psychiatry.10(3). Springer: 251–257.doi:10.1038/sj.mp.4001613.ISBN978-0-387-76760-4.PMID15583703.S2CID21206951.
- Förster E, Jossin Y, Zhao S, Chai X, Frotscher M, Goffinet AM (February 2006). "Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus".The European Journal of Neuroscience.23(4): 901–9.doi:10.1111/j.1460-9568.2006.04612.x.PMID16519655.S2CID25269492.
- Beffert U, Stolt PC, Herz J (March 2004)."Functions of lipoprotein receptors in neurons".Journal of Lipid Research.45(3): 403–9.doi:10.1194/jlr.R300017-JLR200.PMID14657206.
- Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A (August 2005)."Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia".Proceedings of the National Academy of Sciences of the United States of America.102(35): 12578–83.Bibcode:2005PNAS..10212578D.doi:10.1073/pnas.0505394102.PMC1194936.PMID16113080.
- Magdaleno SM, Curran T (December 2001)."Brain development: integrins and the Reelin pathway".Current Biology.11(24): R1032-5.Bibcode:2001CBio...11R1032M.doi:10.1016/S0960-9822(01)00618-2.PMID11747842.S2CID8790079.
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, et al. (September 2000). "Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations".Nature Genetics.26(1): 93–6.doi:10.1038/79246.PMID10973257.S2CID67748801.
External links
[edit]- Overview of all the structural information available in thePDBforUniProt:Q60841(Mouse Reelin) at thePDBe-KB.
- "Gabriella D'Arcangelo".Rutgers University. Archived fromthe originalon 25 July 2008.Retrieved23 August2008.
the scientist who discovered the reelin gene and protein
- Human RELN at WikiGenes
- "Reelin gene expression in mice".Brain Gene Expression Map.St. Jude Children's Research Hospital. Archived fromthe originalon 23 January 2005.Retrieved23 August2008.