Root

Invascular plants,therootsare theorgans of a plantthat are modified to provide anchorage for the plant and take in water and nutrients into the plant body, which allows plants to grow taller and faster.[1]They are most often below the surface of thesoil,but roots can also beaerialor aerating, that is, growing up above the ground or especially above water.[2]
Function
[edit]The major functions of roots areabsorption of water,plant nutritionand anchoring of the plant body to the ground.[3]
Anatomy
[edit]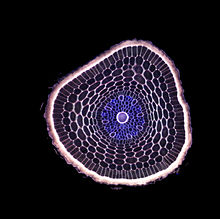
Root morphology is divided into four zones: the root cap, theapical meristem,the elongation zone, and the hair.[4]Theroot capof new roots helps the root penetrate the soil. These root caps are sloughed off as the root goes deeper creating a slimy surface that provides lubrication. The apical meristem behind the root cap produces new root cells that elongate. Then, root hairs form that absorb water and mineral nutrients from the soil.[5]The first root in seed producing plants is theradicle,which expands from the plant embryo after seed germination.
When dissected, the arrangement of the cells in a root isroot hair,epidermis,epiblem,cortex,endodermis,pericycleand, lastly, thevascular tissuein the centre of a root to transport the water absorbed by the root to other places of the plant.[clarification needed]
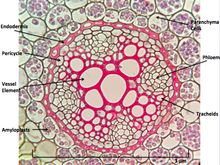
Perhaps the most striking characteristic of roots that distinguishes them from other plant organs such as stem-branches and leaves is that roots have anendogenous[6]origin,i.e.,they originate and develop from an inner layer of the mother axis, such aspericycle.[7]In contrast, stem-branches and leaves areexogenous,i.e.,they start to develop from the cortex, an outer layer.
In response to the concentration of nutrients, roots also synthesisecytokinin,which acts as a signal as to how fast the shoots can grow. Roots often function in storage of food and nutrients. The roots of most vascular plant species enter into symbiosis with certainfungito formmycorrhizae,and a large range of other organisms includingbacteriaalso closely associate with roots.[8]

Root system architecture (RSA)
[edit]
Definition
[edit]In its simplest form, the term root system architecture (RSA) refers to the spatial configuration of a plant's root system. This system can be extremely complex and is dependent upon multiple factors such as the species of the plant itself, the composition of the soil and the availability of nutrients.[9]Root architecture plays the important role of providing a secure supply of nutrients and water as well as anchorage and support.
The configuration of root systems serves to structurally support the plant, compete with other plants and for uptake of nutrients from the soil.[10]Roots grow to specific conditions, which, if changed, can impede a plant's growth. For example, a root system that has developed in dry soil may not be as efficient in flooded soil, yet plants are able to adapt to other changes in the environment, such as seasonal changes.[10]
Terms and components
[edit]The main terms used to classify the architecture of a root system are:[11]
| Branch magnitude | Number of links (exterior or interior) |
| Topology | Pattern of branching (Herringbone,Dichotomous,Radial) |
| Link length | Distance between branches |
| Root angle | Radial angle of a lateral root's base around the parent root's circumference, the angle of a lateral root from its parent root, and the angle an entire system spreads. |
| Link radius | Diameter of root |
All components of the root architecture are regulated through a complex interaction between genetic responses and responses due to environmental stimuli. These developmental stimuli are categorised as intrinsic, the genetic and nutritional influences, or extrinsic, the environmental influences and are interpreted bysignal transduction pathways.[12]
Extrinsic factors affecting root architecture include gravity, light exposure, water and oxygen, as well as the availability or lack of nitrogen, phosphorus, sulphur, aluminium and sodium chloride. The main hormones (intrinsic stimuli) and respective pathways responsible for root architecture development include:
| Auxin | Lateral root formation, maintenance of apical dominance andadventitiousroot formation. |
| Cytokinins | Cytokinins regulate root apical meristem size and promote lateral root elongation. |
| Ethylene | Promotes crown root formation. |
| Gibberellins | Together with ethylene, they promote crown primordia growth and elongation. Together with auxin, they promote root elongation. Gibberellins also inhibit lateral root primordia initiation. |
Growth
[edit]
Early root growth is one of the functions of theapical meristemlocated near the tip of the root. The meristem cells more or less continuously divide, producing more meristem,root capcells (these are sacrificed to protect the meristem), and undifferentiated root cells. The latter become the primary tissues of the root, first undergoing elongation, a process that pushes the root tip forward in the growing medium. Gradually these cells differentiate and mature into specialized cells of the root tissues.[13]
Growth from apical meristems is known asprimary growth,which encompasses all elongation. Secondary growthencompasses all growth in diameter, a major component ofwoody planttissues and many nonwoody plants. For example, storage roots ofsweet potatohave secondary growth but are not woody. Secondary growth occurs at thelateral meristems,namely thevascular cambiumandcork cambium.The former formssecondary xylemandsecondary phloem,while the latter forms theperiderm.
In plants with secondary growth, the vascular cambium, originating between the xylem and the phloem, forms acylinderof tissue along thestemand root.[citation needed]The vascular cambium forms new cells on both the inside and outside of the cambium cylinder, with those on the inside forming secondary xylem cells, and those on the outside forming secondary phloem cells. As secondary xylem accumulates, the "girth" (lateral dimensions) of the stem and root increases. As a result, tissues beyond the secondary phloem including the epidermis and cortex, in many cases tend to be pushed outward and are eventually "sloughed off" (shed).[citation needed]
At this point, the cork cambium begins to form the periderm, consisting of protectivecorkcells. The walls of cork cells containssuberinthickenings, which is an extra cellular complex biopolymer.[14]The suberin thickenings functions by providing a physical barrier, protection against pathogens and by preventing water loss from the surrounding tissues. In addition, it also aids the process of wound healing in plants.[15]It is also postulated that suberin could be a component of the apoplastic barrier (present at the outer cell layers of roots) which prevents toxic compounds from entering the root and reduces radial oxygen loss (ROL) from theaerenchymaduring waterlogging.[16]In roots, the cork cambium originates in thepericycle,a component of the vascular cylinder.[16]
The vascular cambium produces new layers of secondary xylem annually.[citation needed]The xylem vessels are dead at maturity (in some) but are responsible for most water transport through the vascular tissue in stems and roots.

Tree roots usually grow to three times the diameter of the branch spread, only half of which lie underneath the trunk and canopy. The roots from one side of a tree usually supply nutrients to the foliage on the same side. Some families however, such asSapindaceae(themaplefamily), show no correlation between root location and where the root supplies nutrients on the plant.[17]
Regulation
[edit]There is a correlation of roots using the process ofplant perceptionto sense their physical environment to grow,[18]including the sensing of light,[19]and physical barriers. Plants also sense gravity and respond through auxin pathways,[20]resulting ingravitropism.Over time, roots can crack foundations, snap water lines, and lift sidewalks. Research has shown that roots have ability to recognize 'self' and 'non-self' roots in same soil environment.[21]
The correct environment ofair,mineralnutrientsandwaterdirects plant roots to grow in any direction to meet the plant's needs. Roots will shy or shrink away from dry[22]or other poor soil conditions.
Gravitropismdirects roots to grow downward atgermination,the growth mechanism of plants that also causes the shoot to grow upward.[23] Different types of roots such as primary, seminal, lateral and crown are maintained at different gravitropic setpoint angles i.e. the direction in which they grow. Recent research show that root angle in cereal crops such as barley and wheat is regulated by a novel gene called Enhanced Gravitropism 1 (EGT1).[24]
Research indicates that plant roots growing in search of productive nutrition can sense and avoid soil compaction through diffusion of the gasethylene.[25]

Shade avoidance response
[edit]In order to avoid shade, plants utilize a shade avoidance response. When a plant is under dense vegetation, the presence of other vegetation nearby will cause the plant to avoid lateral growth and experience an increase in upward shoot, as well as downward root growth. In order to escape shade, plants adjust their root architecture, most notably by decreasing the length and amount of lateral roots emerging from the primary root. Experimentation of mutant variants ofArabidopsis thalianafound that plants sense the Red to Far Red light ratio that enters the plant through photoreceptors known asphytochromes.[26]Nearby plant leaves will absorb red light and reflect far-red light, which will cause the ratio red to far red light to lower. The phytochrome PhyA that senses this Red to Far Red light ratio is localized in both the root system as well as the shoot system of plants, but through knockout mutant experimentation, it was found that root localized PhyA does not sense the light ratio, whether directly or axially, that leads to changes in the lateral root architecture.[26]Research instead found that shoot localized PhyA is the phytochrome responsible for causing these architectural changes of the lateral root. Research has also found that phytochrome completes these architectural changes through the manipulation of auxin distribution in the root of the plant.[26]When a low enough Red to Far Red ratio is sensed by PhyA, the phyA in the shoot will be mostly in its active form.[27]In this form, PhyA stabilize thetranscription factorHY5 causing it to no longer be degraded as it is when phyA is in its inactive form. This stabilized transcription factor is then able to be transported to the roots of the plant through thephloem,where it proceeds to induce its own transcription as a way to amplify its signal. In the roots of the plant HY5 functions to inhibit an auxin response factor known as ARF19, a response factor responsible for the translation of PIN3 and LAX3, two well known auxin transportingproteins.[27]Thus, through manipulation of ARF19, the level and activity ofauxintransporters PIN3 and LAX3 is inhibited.[27]Once inhibited, auxin levels will be low in areas where lateral root emergence normally occurs, resulting in a failure for the plant to have the emergence of the lateral root primordium through the rootpericycle.With this complex manipulation of Auxin transport in the roots, lateral root emergence will be inhibited in the roots and the root will instead elongate downwards, promoting vertical plant growth in an attempt to avoid shade.[26][27]
Research of Arabidopsis has led to the discovery of how this auxin mediated root response works. In an attempt to discover the role thatphytochromeplays in lateral root development, Salisbury et al. (2007) worked withArabidopsis thalianagrown on agar plates. Salisbury et al. used wild type plants along with varying protein knockout and gene knockout Arabidopsis mutants to observe the results these mutations had on the root architecture, protein presence, and gene expression. To do this, Salisbury et al. used GFP fluorescence along with other forms of both macro and microscopic imagery to observe any changes various mutations caused. From these research, Salisbury et al. were able to theorize that shoot located phytochromes alter auxin levels in roots, controlling lateral root development and overall root architecture.[26]In the experiments of van Gelderen et al. (2018), they wanted to see if and how it is that the shoot ofA. thalianaalters and affects root development and root architecture. To do this, they tookArabidopsisplants, grew them inagar gel,and exposed the roots and shoots to separate sources of light. From here, they altered the different wavelengths of light the shoot and root of the plants were receiving and recorded the lateral root density, amount of lateral roots, and the general architecture of the lateral roots. To identify the function of specific photoreceptors, proteins, genes, and hormones, they utilized variousArabidopsisknockout mutants and observed the resulting changes in lateral roots architecture. Through their observations and various experiments, van Gelderen et al. were able to develop a mechanism for how root detection of Red to Far-red light ratios alter lateral root development.[27]
Types
[edit]A true root system consists of aprimary rootandsecondary roots(orlateral roots).
- the diffuse root system: the primary root is not dominant; the whole root system is fibrous and branches in all directions. Most common inmonocots.The main function of the fibrous root is to anchor the plant.
Specialized
[edit]







The roots, or parts of roots, of many plant species have become specialized to serve adaptive purposes besides the two primary functions[clarification needed],described in the introduction.
- Adventitious rootsarise out-of-sequence from the more usual root formation of branches of a primary root, and instead originate from the stem, branches, leaves, or old woody roots. They commonly occur inmonocotsand pteridophytes, but also in manydicots,such asclover(Trifolium),ivy(Hedera),strawberry(Fragaria) andwillow(Salix). Most aerial roots and stilt roots are adventitious. In some conifers adventitious roots can form the largest part of the root system. Adventitious root formation is enhanced in many plant species during (partial) submergence, to increase gas exchange and storage of gases like oxygen.[28]Distinct types of adventitious roots can be classified and are dependent on morphology, growth dynamics and function.[29][30]
- Aerating roots(orknee rootorkneeorpneumatophores): roots rising above the ground, especially above water such as in somemangrovegenera (Avicennia,Sonneratia). In some plants likeAvicenniathe erect roots have a large number of breathing pores for exchange of gases.
- Aerial roots:roots entirely above the ground, such as in ivy (Hedera) or inepiphyticorchids.Many aerial roots are used to receive water and nutrient intake directly from the air – from fogs, dew or humidity in the air.[31]Some rely on leaf systems to gather rain or humidity and even store it in scales or pockets. Other aerial roots, such asmangroveaerial roots, are used for aeration and not for water absorption. Other aerial roots are used mainly for structure, functioning as prop roots, as inmaizeor anchor roots or as the trunk instrangler fig.In some Epiphytes – plants living above the surface on other plants, aerial roots serve for reaching to water sources or reaching the surface, and then functioning as regular surface roots.[31]
- Canopy roots/arboreal roots:roots that form when tree branches support mats of epiphytes and detritus, which hold water and nutrients in the canopy. They grow out into these mats, likely to utilize the available nutrients and moisture.[32]
- Contractile roots:roots that pull bulbs or corms ofmonocots,such ashyacinthandlily,and sometaproots,such asdandelion,deeper in the soil through expanding radially and contracting longitudinally. They have a wrinkled surface.[33]
- Coarse roots:roots that have undergone secondary thickening and have a woody structure. These roots have some ability to absorb water and nutrients, but their main function is transport and to provide a structure to connect the smaller diameter, fine roots to the rest of the plant.
- Dimorphic root systems:roots with two distinctive forms for two separate functions
- Fine roots:typically primary roots <2 mm diameter that have the function of water and nutrient uptake. They are often heavily branched and support mycorrhizas. These roots may be short lived, but are replaced by the plant in an ongoing process of root 'turnover'.
- Haustorial roots:roots of parasitic plants that can absorb water and nutrients from another plant, such as inmistletoe(Viscum album) anddodder.
- Propagative roots:roots that form adventitious buds that develop into aboveground shoots, termedsuckers,which form new plants, as inCanada thistle,cherryand many others.
- Proteoid rootsor cluster roots: dense clusters of rootlets of limited growth that develop under lowphosphateor lowironconditions inProteaceaeand some plants from the following familiesBetulaceae,Casuarinaceae,Elaeagnaceae,Moraceae,FabaceaeandMyricaceae.
- Stilt roots:adventitious support roots, common amongmangroves.They grow down from lateral branches, branching in the soil.
- Storage roots:roots modified for storage of food or water, such ascarrotsandbeets.They include sometaprootsand tuberous roots.
- Structural roots:large roots that have undergone considerable secondary thickening and provide mechanical support to woody plants and trees.
- Surface roots:roots that proliferate close below the soil surface, exploiting water and easily available nutrients. Where conditions are close to optimum in the surface layers of soil, the growth of surface roots is encouraged and they commonly become the dominant roots.
- Tuberous roots:fleshy and enlarged lateral roots for food or water storage, e.g.sweet potato.A type of storage root distinct from taproot.
- Photosynthetic roots:roots that are green and photosynthesize, providing sugar to the plant. They are similar tophylloclades.Several orchids have these, such asDendrophylaxandTaeniophyllum.
- Root nodules:roots that harbor nitrogen-fixing soil bacteria. These are often very short and rounded. Root nodules are found in virtually alllegumes.
- Coralloid roots: similar to root nodules, these provide nitrogen to the plant. They are often larger than nodules, branched, and located at or near the soil surface, and harbor nitrogen-fixingcyanobacteria.They are only found incycads.
Depths
[edit]
The distribution of vascular plant roots within soil depends on plant form, the spatial and temporal availability of water and nutrients, and the physical properties of the soil. The deepest roots are generally found in deserts and temperate coniferous forests; the shallowest in tundra, boreal forest and temperate grasslands. The deepest observed living root, at least 60 metres (200 ft) below the ground surface, was observed during the excavation of an open-pit mine in Arizona, US. Some roots can grow as deep as the tree is high. The majority of roots on most plants are however found relatively close to the surface where nutrient availability and aeration are more favourable for growth. Rooting depth may be physically restricted by rock or compacted soil close below the surface, or by anaerobic soil conditions.
Records
[edit]
| Species | Location | Maximum rooting depth (m) | References[34][35] |
|---|---|---|---|
| Boscia albitrunca | Kalahari desert | 68 | Jennings (1974) |
| Juniperus monosperma | Colorado Plateau | 61 | Cannon (1960) |
| Eucalyptussp. | Australian forest | 61 | Jennings (1971) |
| Acacia erioloba | Kalahari desert | 60 | Jennings (1974) |
| Prosopis juliflora | Arizona desert | 53.3 | Phillips (1963) |
Evolutionary history
[edit]The fossil record of roots—or rather, infilled voids where roots rotted after death—spans back to the lateSilurian,about 430 million years ago.[36]Their identification is difficult, because casts and molds of roots are so similar in appearance to animal burrows. They can be discriminated using a range of features.[37]The evolutionary development of roots likely happened from the modification of shallowrhizomes(modified horizontal stems) which anchored primitive vascular plants combined with the development of filamentous outgrowths (calledrhizoids) which anchored the plants and conducted water to the plant from the soil.[38]
Environmental interactions
[edit]
Light has been shown to have some impact on roots, but its not been studied as much as the effect of light on other plant systems. Early research in the 1930s found that light decreased the effectiveness ofIndole-3-acetic acidon adventitious root initiation. Studies of the pea in the 1950s shows that lateral root formation was inhibited by light, and in the early 1960s researchers found that light could induce positivegravitropicresponses in some situations. The effects of light on root elongation has been studied formonocotyledonousanddicotyledonousplants, with the majority of studies finding that light inhibited root elongation, whether pulsed or continuous. Studies ofArabidopsisin the 1990s showed negativephototropismand inhibition of the elongation of root hairs in light sensed byphyB.[39]
Certain plants, namelyFabaceae,formroot nodulesin order to associate and form a symbiotic relationship with nitrogen-fixing bacteria calledrhizobia.Owing to the high energy required to fix nitrogen from the atmosphere, the bacteria take carbon compounds from the plant to fuel the process. In return, the plant takes nitrogen compounds produced from ammonia by the bacteria.[40]
Soil temperature is a factor that effectsroot initiationand length. Root length is usually impacted more dramatically by temperature than overall mass, where cooler temperatures tend to cause more lateral growth because downward extension is limited by cooler temperatures at subsoil levels. Needs vary by plant species, but in temperate regions cool temperatures may limit root systems. Cool temperature species likeoats,rapeseed,rye,wheatfare better in lower temperatures than summerannualslikemaizeandcotton.Researchers have found that plants like cotton develop wider and shortertaprootsin cooler temperatures. The first root originating from the seed usually has a wider diameter than root branches, so smaller root diameters are expected if temperatures increase root initiation. Root diameter also decreases when the root elongates.[41]
Plant interactions
[edit]Plants can interact with one another in their environment through their root systems. Studies have demonstrated that plant-plant interaction occurs among root systems via the soil as a medium. Researchers have tested whether plants growing in ambient conditions would change their behavior if a nearby plant was exposed to drought conditions.[42] Since nearby plants showed no changes instomatalaperture researchers believe the drought signal spread through the roots and soil, not through the air as a volatile chemical signal.[43]
Soil interactions
[edit]Soil microbiota can suppress both disease and beneficial root symbionts (mycorrhizal fungi are easier to establish in sterile soil). Inoculation with soil bacteria can increase internode extension, yield and quicken flowering. The migration of bacteria along the root varies with natural soil conditions. For example, research has found that the root systems of wheat seeds inoculated withAzotobactershowed higher populations in soils favorable toAzotobactergrowth. Some studies have been unsuccessful in increasing the levels of certain microbes (such asP. fluorescens) in natural soil without prior sterilization.[44]
Grass root systems are beneficial at reducingsoil erosionby holding the soil together.Perennialgrasses that grow wild in rangelands contribute organic matter to the soil when their old roots decay after attacks by beneficialfungi,protozoa,bacteria, insects and worms release nutrients.[5]
Scientists have observed significant diversity of the microbial cover of roots at around 10 percent of three week old root segments covered. On younger roots there was even low coverage, but even on 3-month-old roots the coverage was only around 37%. Before the 1970s, scientists believed that the majority of the root surface was covered by microorganisms.[5]
Nutrient absorption
[edit]Researchers studyingmaizeseedlings found that calcium absorption was greatest in theapicalroot segment, and potassium at the base of the root. Along other root segments absorption was similar. Absorbed potassium is transported to the root tip, and to a lesser extent other parts of the root, then also to the shoot and grain. Calcium transport from the apical segment is slower, mostly transported upward and accumulated in stem and shoot.[45]
Researchers found that partial deficiencies of K or P did not change thefatty acidcomposition ofphosphatidyl cholineinBrassica napus L.plants. Calcium deficiency did, on the other hand, lead to a marked decline ofpolyunsaturatedcompounds that would be expected to have negative impacts for integrity of the plantmembrane,that could effect some properties like its permeability, and is needed for theionuptake activity of the root membranes.[46]
Economic importance
[edit]

The termroot cropsrefers to any edible underground plant structure, but many root crops are actually stems, such aspotatotubers. Edible roots includecassava,sweet potato,beet,carrot,rutabaga,turnip,parsnip,radish,yamandhorseradish.Spices obtained from roots includesassafras,angelica,sarsaparillaandlicorice.
Sugar beetis an important source of sugar.Yamroots are a source ofestrogencompounds used inbirth control pills.The fishpoisonandinsecticiderotenoneis obtained from roots ofLonchocarpusspp. Important medicines from roots areginseng,aconite,ipecac,gentianandreserpine.Several legumes that have nitrogen-fixing root nodules are used as green manure crops, which provide nitrogen fertilizer for other crops when plowed under. Specializedbald cypressroots, termed knees, are sold as souvenirs, lamp bases and carved into folk art.Native Americansused the flexible roots ofwhite sprucefor basketry.
Treeroots can heave and destroyconcretesidewalks and crush or clog buried pipes.[47]The aerial roots ofstrangler fighave damaged ancientMayantemplesinCentral Americaand the temple ofAngkor WatinCambodia.
Trees stabilize soil on a slope prone tolandslides.Theroot hairswork as an anchor on the soil.
Vegetative propagationof plants via cuttings depends on adventitious root formation. Hundreds of millions of plants are propagated viacuttingsannually includingchrysanthemum,poinsettia,carnation,ornamentalshrubsand manyhouseplants.
Roots can also protect the environment by holding the soil to reduce soil erosion. This is especially important in areas such assand dunes.

See also
[edit]- Absorption of water
- Cypress knee
- Drought rhizogenesis
- Fibrous root system
- Mycorrhiza– root symbiosis in which individual hyphae extending from the mycelium of a fungus colonize the roots of a host plant.
- Mycorrhizal network
- Plant physiology
- Rhizosphere– region of soil around the root influenced by root secretions and microorganisms present
- Root cutting
- Rooting powder
- Stolon
- Tanada effect
- Taproot
References
[edit]- ^Harley Macdonald & Donovan Stevens (3 September 2019).Biotechnology and Plant Biology.EDTECH. pp. 141–.ISBN978-1-83947-180-3.
- ^Nguyen, Linh Thuy My; Hoang, Hanh Thi; Choi, Eunho; Park, Pil Sun (2023-07-05)."Distribution of mangroves with different aerial root morphologies at accretion and erosion sites in Ca Mau Province, Vietnam".Estuarine, Coastal and Shelf Science.287:108324.Bibcode:2023ECSS..28708324N.doi:10.1016/j.ecss.2023.108324.ISSN0272-7714.
- ^"Plant parts=Roots".University of Illinois Extension.
- ^Yaacov Okon (24 November 1993).Azospirillum/Plant Associations.CRC Press. pp. 77–.ISBN978-0-8493-4925-6.
- ^abc"Backyard Gardener: Understanding Plant Roots".University of Arizona Cooperative Extension.
- ^Gangulee HC, Das KS, Datta CT, Sen S.College Botany.Vol. 1. Kolkata: New Central Book Agency.
- ^Dutta AC, Dutta TC.BOTANY For Degree Students(6th ed.). Oxford University Press.
- ^Sheldrake, Merlin (2020).Entangled Life.Bodley Head. p. 148.ISBN978-1847925206.
- ^Malamy JE (2005)."Intrinsic and environmental response pathways that regulate root system architecture".Plant, Cell & Environment.28(1): 67–77.doi:10.1111/j.1365-3040.2005.01306.x.PMID16021787.
- ^abCaldwell MM, Dawson TE, Richards JH (January 1998). "Hydraulic lift: consequences of water efflux from the roots of plants".Oecologia.113(2): 151–161.Bibcode:1998Oecol.113..151C.doi:10.1007/s004420050363.PMID28308192.S2CID24181646.
- ^Fitter AH (1991). "The ecological significance of root system architecture: an economic approach". In Atkinson D (ed.).Plant Root Growth: An Ecological Perspective.Blackwell. pp. 229–243.
- ^Malamy JE, Ryan KS (November 2001)."Environmental regulation of lateral root initiation in Arabidopsis".Plant Physiology.127(3): 899–909.doi:10.1104/pp.010406.PMC129261.PMID11706172.
- ^Russell PJ, Hertz PE, McMillan B (2013).Biology: The Dynamic Science.Cengage Learning. p. 750.ISBN978-1-285-41534-5.Archivedfrom the original on 2018-01-21.Retrieved2017-04-24.
- ^"Suberin – an overview | ScienceDirect Topics".www.sciencedirect.com.Retrieved2021-08-31.
- ^"Suberin Form & Function – Mark Bernards – Western University".www.uwo.ca.Retrieved2021-08-31.
- ^abWatanabe, Kohtaro; Nishiuchi, Shunsaku; Kulichikhin, Konstantin; Nakazono, Mikio (2013)."Does suberin accumulation in plant roots contribute to waterlogging tolerance?".Frontiers in Plant Science.4:178.doi:10.3389/fpls.2013.00178.ISSN1664-462X.PMC3683634.PMID23785371.
- ^van den Driessche, R. (1974-07-01)."Prediction of mineral nutrient status of trees by foliar analysis".The Botanical Review.40(3): 347–394.Bibcode:1974BotRv..40..347V.doi:10.1007/BF02860066.ISSN1874-9372.S2CID29919924– via Springer.
- ^Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, et al. (February 2007)."Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots".Proceedings of the National Academy of Sciences of the United States of America.104(9): 3639–44.Bibcode:2007PNAS..104.3639N.doi:10.1073/pnas.0607703104.PMC1802001.PMID17360695.
- ^UV-B light sensing mechanism discovered in plant roots,San Francisco State University, December 8, 2008
- ^Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (April 1999)."AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues".The EMBO Journal.18(8): 2066–73.doi:10.1093/emboj/18.8.2066.PMC1171291.PMID10205161.
- ^Hodge A (June 2009)."Root decisions".Plant, Cell & Environment.32(6): 628–40.doi:10.1111/j.1365-3040.2008.01891.x.PMID18811732.
- ^Carminati A, Vetterlein D, Weller U, Vogel HJ, Oswald SE (2009). "When roots lose contact".Vadose Zone Journal.8(3): 805–809.Bibcode:2009VZJ.....8..805C.doi:10.2136/vzj2008.0147.S2CID128600212.
- ^Chen R, Rosen E, Masson PH (June 1999)."Gravitropism in higher plants".Plant Physiology.120(2): 343–50.doi:10.1104/pp.120.2.343.PMC1539215.PMID11541950.
- ^Fusi, Riccardo; Rosignoli, Serena; Lou, Haoyu; Sangiorgi, Giuseppe; Bovina, Riccardo; Pattem, Jacob K.; Borkar, Aditi N.; Lombardi, Marco; Forestan, Cristian; Milner, Sara G.; Davis, Jayne L.; Lale, Aneesh; Kirschner, Gwendolyn K.; Swarup, Ranjan; Tassinari, Alberto; Pandey, Bipin K.; York, Larry M.; Atkinson, Brian S.; Sturrock, Craig J.; Mooney, Sacha J.; Hochholdinger, Frank; Tucker, Matthew R.; Himmelbach, Axel; Stein, Nils; Mascher, Martin; Nagel, Kerstin A.; De Gara, Laura; Simmonds, James; Uauy, Cristobal; Tuberosa, Roberto; Lynch, Jonathan P.; Yakubov, Gleb E.; Bennett, Malcolm J.; Bhosale, Rahul; Salvi, Silvio (2 August 2022)."Root angle is controlled by EGT1 in cereal crops employing an antigravitropic mechanism".Proceedings of the National Academy of Sciences.119(31): e2201350119.Bibcode:2022PNAS..11901350F.doi:10.1073/pnas.2201350119.PMC9351459.PMID35881796.S2CID251104211.
- ^Pandey, Bipin K.; Huang, Guoqiang; Bhosale, Rahul; Hartman, Sjon; Sturrock, Craig J.; Jose, Lottie; Martin, Olivier C.; Karady, Michal; Voesenek, Laurentius A. C. J.; Ljung, Karin; Lynch, Jonathan P. (2021-01-15)."Plant roots sense soil compaction through restricted ethylene diffusion".Science.371(6526): 276–280.Bibcode:2021Sci...371..276P.doi:10.1126/science.abf3013.hdl:1874/418726.ISSN0036-8075.PMID33446554.S2CID231606782.
- ^abcdeSalisbury FJ, Hall A, Grierson CS, Halliday KJ (May 2007)."Phytochrome coordinates Arabidopsis shoot and root development".The Plant Journal.50(3): 429–38.doi:10.1111/j.1365-313x.2007.03059.x.PMID17419844.
- ^abcdevan Gelderen K, Kang C, Paalman R, Keuskamp D, Hayes S, Pierik R (January 2018)."Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor".The Plant Cell.30(1): 101–116.doi:10.1105/tpc.17.00771.PMC5810572.PMID29321188.
- ^Ayi, Qiaoli; Zeng, Bo; Liu, Jianhui; Li, Siqi; van Bodegom, Peter M.; Cornelissen, Johannes H. C. (October 2016)."Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants".Annals of Botany.118(4): 675–683.doi:10.1093/aob/mcw051.PMC5055620.PMID27063366.
- ^Lin, Chen; Ogorek, Lucas León Peralta; Liu, Dan; Pedersen, Ole; Sauter, Margret (11 January 2023)."A quantitative trait locus conferring flood tolerance to deepwater rice regulates the formation of two distinct types of aquatic adventitious roots".New Phytologist.238(4): 1403–1419.doi:10.1111/nph.18678.PMID36519256.S2CID254769152.
- ^Maric, Aida; Hartman, Sjon (11 March 2023)."The leaf sheath promotes prolonged flooding protection by giving rise to specialized adventitious roots".New Phytologist.238(4): 1337–1339.doi:10.1111/nph.18824.PMID36905344.S2CID257438007.
- ^abNowak EJ, Martin CE (1997)."Physiological and anatomical responses to water deficits in the CAM epiphyteTillandsia ionantha(Bromeliaceae) ".International Journal of Plant Sciences.158(6): 818–826.doi:10.1086/297495.hdl:1808/9858.JSTOR2475361.S2CID85888916.
- ^Nadkarni NM (November 1981). "Canopy roots: convergent evolution in rainforest nutrient cycles".Science.214(4524): 1023–4.Bibcode:1981Sci...214.1023N.doi:10.1126/science.214.4524.1023.PMID17808667.S2CID778003.
- ^Pütz N (2002). "Contractile roots". In Waisel Y., Eshel A., Kafkafi U. (eds.).Plant roots: The hidden half(3rd ed.). New York: Marcel Dekker. pp. 975–987.
- ^Canadell J, Jackson RB, Ehleringer JB, Mooney HA, Sala OE, Schulze ED (December 1996). "Maximum rooting depth of vegetation types at the global scale".Oecologia.108(4): 583–595.Bibcode:1996Oecol.108..583C.doi:10.1007/BF00329030.PMID28307789.S2CID2092130.
- ^Stonea EL, Kaliszb PJ (1 December 1991). "On the maximum extent of tree roots".Forest Ecology and Management.46(1–2): 59–102.Bibcode:1991ForEM..46...59S.doi:10.1016/0378-1127(91)90245-Q.
- ^Retallack GJ (1986)."The fossil record of soils"(PDF).In Wright VP (ed.).Paleosols: their Recognition and Interpretation.Oxford: Blackwell. pp. 1–57.Archived(PDF)from the original on 2017-01-07.
- ^Hillier R, Edwards D, Morrissey LB (2008). "Sedimentological evidence for rooting structures in the Early Devonian Anglo–Welsh Basin (UK), with speculation on their producers".Palaeogeography, Palaeoclimatology, Palaeoecology.270(3–4): 366–380.Bibcode:2008PPP...270..366H.doi:10.1016/j.palaeo.2008.01.038.
- ^Amram Eshel; Tom Beeckman (17 April 2013).Plant Roots: The Hidden Half, Fourth Edition.CRC Press. pp. 1–.ISBN978-1-4398-4649-0.
- ^Kurata, Tetsuya (1997). "Light-stimulated root elongation in Arabidopsis thaliana".Journal of Plant Physiology.151(3): 345–351.Bibcode:1997JPPhy.151..346K.doi:10.1016/S0176-1617(97)80263-5.hdl:2115/44841.
- ^Postgate, J. (1998).Nitrogen Fixation(3rd ed.). Cambridge, UK: Cambridge University Press.
- ^Lal, Rattan (2006).Encyclopedia of Soil Science.CRC Press.ISBN978-0-8493-5054-2.
- ^Chamovitz, Daniel. (21 November 2017).What a plant knows: a field guide to the senses.Farrar, Straus and Giroux.ISBN9780374537128.OCLC1041421612.
- ^Falik O, Mordoch Y, Ben-Natan D, Vanunu M, Goldstein O, Novoplansky A (July 2012)."Plant responsiveness to root-root communication of stress cues".Annals of Botany.110(2): 271–80.doi:10.1093/aob/mcs045.PMC3394639.PMID22408186.
- ^Bowen GD, Rovira AD (1976). "Microbial Colonization of Plant Roots".Annu. Rev. Phytopathol.14:121–144.doi:10.1146/annurev.py.14.090176.001005.
- ^Plant Roots and their Environment.Elsevier. 1988. p. 17.
- ^Plant Roots and their Environment.Elsevier. 1988. p. 25.
- ^Zahniser, David (2008-02-21)."City to pass the bucks on sidewalks?".The Los Angeles Times.Retrieved2023-03-30.
Further reading
[edit]- Baldocchi DD, Xu L (October 2007). "What limits evaporation from Mediterranean oak woodlands–The supply of moisture in the soil, physiological control by plants or the demand by the atmosphere?".Advances in Water Resources.30(10): 2113–22.Bibcode:2007AdWR...30.2113B.doi:10.1016/j.advwatres.2006.06.013.
- Brundrett, M. C. (2002)."Coevolution of roots and mycorrhizas of land plants".New Phytologist.154(2): 275–304.doi:10.1046/j.1469-8137.2002.00397.x.PMID33873429.
- Clark L (2004)."Primary Root Structure and Development – lecture notes"(PDF).Archived fromthe original(PDF)on 3 January 2006.
- Coutts MP (1987). "Developmental processes in tree root systems".Canadian Journal of Forest Research.17(8): 761–767.doi:10.1139/x87-122.
- Raven JA, Edwards D (2001). "Roots: evolutionary origins and biogeochemical significance".Journal of Experimental Botany.52(Suppl 1): 381–401.doi:10.1093/jxb/52.suppl_1.381.PMID11326045.
- Schenk HJ, Jackson RB (2002). "The global biogeography of roots".Ecological Monographs.72(3): 311–328.doi:10.2307/3100092.JSTOR3100092.
- Sutton RF, Tinus RW (1983). "Root and root system terminology".Forest Science Monograph.24:137.
- Phillips WS (1963). "Depth of roots in soil".Ecology.44(2): 424.Bibcode:1963Ecol...44..424P.doi:10.2307/1932198.JSTOR1932198.
- Caldwell MM, Dawson TE, Richards JH (1998). "Hydraulic lift: consequences of water efflux from the roots of plants".Oecologia.113(2): 151–161.Bibcode:1998Oecol.113..151C.doi:10.1007/s004420050363.PMID28308192.S2CID24181646.
