Route of administration

Inpharmacologyandtoxicology,aroute of administrationis the way by which adrug,fluid, poison, or other substance is taken into the body.[1]
Routes of administration are generally classified by the location at which the substance is applied. Common examples includeoralandintravenousadministration. Routes can also be classified based on where the target of action is. Action may betopical(local),enteral(system-wide effect, but delivered through thegastrointestinal tract), orparenteral(systemic action, but is delivered by routes other than the GI tract). Route of administration anddosage formare aspects ofdrug delivery.
Classification[edit]
Routes of administration are usually classified by application location (or exposition).
The route or course the active substance takes from application location to the location where it has its target effect is usually rather a matter ofpharmacokinetics(concerning the processes of uptake, distribution, and elimination of drugs). Exceptions include thetransdermalor transmucosal routes, which are still commonly referred to asroutes of administration.
The location of the target effect of active substances are usually rather a matter ofpharmacodynamics(concerning e.g. the physiological effects of drugs[2]). An exception istopical administration,which generally means that both the application location and the effect thereof is local.[3]
Topical administrationis sometimes defined as both a local application location and localpharmacodynamiceffect,[3]and sometimes merely as a local application location regardless of location of the effects.[4][5]
By application location[edit]
Enteral/gastrointestinal route[edit]
Through thegastrointestinal tractis sometimes termedenteral or enteric administration(literally meaning 'through theintestines').Enteral/enteric administrationusually includesoral[6](through themouth) andrectal(into therectum)[6]administration, in the sense that these are taken up by the intestines. However, uptake of drugs administered orally may also occur already in thestomach,and as suchgastrointestinal(along thegastrointestinal tract) may be a more fitting term for this route of administration. Furthermore, some application locations often classified asenteral,such assublingual[6](under the tongue) andsublabialorbuccal(between the cheek and gums/gingiva), are taken up in the proximal part of thegastrointestinal tractwithout reaching the intestines. Strictlyenteral administration(directly into the intestines) can be used for systemic administration, as well as local (sometimes termedtopical), such as in a contrastenema,whereby contrast media are infused into the intestines for imaging. However, for the purposes of classification based on location of effects, the term enteral is reserved for substances with systemic effects.

Many drugs astablets,capsules,or drops are taken orally. Administration methods directly into the stomach include those bygastric feeding tubeorgastrostomy.Substances may also be placed into thesmall intestines,as with aduodenalfeeding tube andenteral nutrition.Enteric coated tablets are designed to dissolve in the intestine, not the stomach, because the drug present in the tablet causes irritation in the stomach.
Therectalroute is an effective route of administration for many medications, especially those used at theend of life.[7][8][9][10][11][12][13]The walls of the rectum absorb many medications quickly and effectively.[14]Medications delivered to the distal one-third of the rectum at least partially avoid the "first pass effect"through the liver, which allows for greaterbio-availabilityof many medications than that of the oral route. Rectalmucosais highlyvascularizedtissue that allows for rapid and effective absorption of medications.[15]Asuppositoryis a soliddosage formthat fits forrectal administration.Inhospice care,a specialized rectalcatheter,designed to provide comfortable and discreet administration of ongoing medications provides a practical way to deliver and retain liquid formulations in thedistalrectum, giving health practitioners a way to leverage the established benefits of rectal administration. TheMurphy dripis an example of rectal infusion.
Parenteral route[edit]

The parenteral route is any route that is notenteral(par-+enteral).
Parenteral administration can be performed byinjection,that is, using a needle (usually ahypodermic needle) and asyringe,[16]or by the insertion of anindwelling catheter.
Locations of application of parenteral administration include:
- Central nervous system:
- Epidural(synonym: peridural) (injection or infusion into theepidural space), e.g. epidural anesthesia.
- Intracerebral (into the cerebrum) administration by direct injection into the brain. Used in experimental research of chemicals[17]and as a treatment for malignancies of the brain.[18]The intracerebral route can also interrupt the blood brain barrier from holding up against subsequent routes.[19]
- Intracerebroventricular(into the cerebral ventricles) administration into the ventricular system of the brain. One use is as a last line of opioid treatment for terminal cancer patients with intractablecancer pain.[20]

- Epicutaneous (application onto the skin). It can be used both for local effect as in allergy testing and typicallocal anesthesia,as well assystemiceffects when the active substance diffuses through skin in atransdermalroute.
- Sublingual and buccal medication administration is a way of giving someone medicine orally (by mouth).Sublingual administrationis when medication is placed under the tongue to be absorbed by the body. The word "sublingual" means "under the tongue." Buccal administration involves placement of the drug between the gums and the cheek. These medications can come in the form of tablets, films, or sprays. Many drugs are designed for sublingual administration, including cardiovascular drugs, steroids, barbiturates, opioid analgesics with poor gastrointestinal bioavailability, enzymes and, increasingly, vitamins and minerals.
- Extra-amniotic administration,between theendometriumandfetal membranes.
- Nasal administration(through the nose) can be used for topically acting substances, as well as forinsufflationof e.g.decongestantnasal sprays to be taken up along therespiratory tract.Such substances are also calledinhalational,e.g.inhalational anesthetics.
- Intra-arterial (into anartery), e.g.vasodilatordrugs in the treatment ofvasospasmandthrombolytic drugsfor treatment ofembolism.
- Intra-articular,into a joint space. It is generally performed byjoint injection.It is mainly used for symptomatic relief inosteoarthritis.
- Intracardiac(into the heart), e.g.adrenalineduringcardiopulmonary resuscitation(no longer commonly performed).
- Intracavernous injection,an injection into the base of thepenis.
- Intradermal,(into the skin itself) is used forskin testingsomeallergens,and also formantoux testfortuberculosis.
- Intralesional (into a skin lesion), is used for local skin lesions, e.g. acne medication.
- Intramuscular(into amuscle), e.g. manyvaccines,antibiotics, and long-term psychoactive agents. Recreationally the colloquial term 'muscling' is used.[21]

- Intraocular, into the eye, e.g., some medications forglaucomaoreye neoplasms.
- Intraosseous infusion(into thebonemarrow) is, in effect, an indirect intravenous access because the bone marrow drains directly into the venous system. This route is occasionally used for drugs and fluids in emergency medicine and pediatrics when intravenous access is difficult.
- Intraperitoneal,(infusion or injection into theperitoneum) e.g.peritoneal dialysis.
- Intrathecal(into the spinal canal) is most commonly used for spinalanesthesiaandchemotherapy.
- Intrauterine.
- Intravaginal administration,in thevagina.
- Intravenous(into avein), e.g. many drugs,total parenteral nutrition.
- Intravesicalinfusion is into the urinary bladder.
- Intravitreal,through the eye.
- Subcutaneous (under the skin).[22]This generally takes the form ofsubcutaneous injection,e.g. withinsulin.Skin poppingis a slang term that includes subcutaneous injection, and is usually used in association withrecreational drugs.In addition to injection, it is also possible to slowly infuse fluids subcutaneously in the form ofhypodermoclysis.
- Transdermal(diffusion through the intact skin for systemic rather than topical distribution), e.g.transdermal patchessuch asfentanylin pain therapy,nicotinepatches for treatment ofaddictionandnitroglycerinefor treatment ofangina pectoris.
- Perivascular administration(perivascular medical devices and perivascular drug delivery systems are conceived for local application around a blood vessel during open vascular surgery).[23]
- Transmucosal (diffusion through a mucous membrane), e.g.insufflation(snorting) ofcocaine,sublingual,i.e. under the tongue,sublabial,i.e. between the lips andgingiva,nitroglycerine,vaginal suppositories.
Topical route[edit]
The definition of the topical route of administration sometimes states that both the application location and thepharmacodynamiceffect thereof is local.[3]
In other cases,topicalis defined as applied to a localized area of the body or to the surface of a body part regardless of the location of the effect.[4][5]By this definition,topical administrationalso includestransdermalapplication, where the substance is administered onto the skin but isabsorbedinto the body to attainsystemicdistribution.
If defined strictly as having local effect, the topical route of administration can also includeenteral administrationof medications that are poorly absorbable by thegastrointestinal tract.One such medication is the antibioticvancomycin,which cannot be absorbed in the gastrointestinal tract and is used orally only as a treatment forClostridium difficilecolitis.[24]
Choice of routes[edit]
The reason for choice of routes of drug administration are governing by various factors:
- Physical and chemical properties of the drug. The physical properties are solid, liquid and gas. The chemical properties are solubility, stability, pH, irritancy etc.
- Site of desired action: the action may be localised and approachable or generalised and not approachable.
- Rate of extent of absorption of the drug from different routes.
- Effect of digestive juices and the first pass metabolism of drugs.
- Condition of the patient.
In acute situations, inemergency medicineandintensive care medicine,drugs are most often given intravenously. This is the most reliable route, as in acutely ill patients the absorption of substances from the tissues and from the digestive tract can often be unpredictable due to altered blood flow or bowel motility.
Convenience[edit]
Enteral routes are generally the most convenient for the patient, as no punctures orsterileprocedures are necessary. Enteral medications are therefore often preferred in the treatment of chronic disease. However, some drugs can not be used enterally because their absorption in the digestive tract is low or unpredictable. Transdermal administration is a comfortable alternative; there are, however, only a few drug preparations that are suitable for transdermal administration.
Desired target effect[edit]
Identical drugs can produce different results depending on the route of administration. For example, some drugs are not significantly absorbed into the bloodstream from the gastrointestinal tract and their action after enteral administration is therefore different from that after parenteral administration. This can be illustrated by the action ofnaloxone(Narcan), an antagonist ofopiatessuch asmorphine.Naloxone counteracts opiate action in thecentral nervous systemwhen given intravenously and is therefore used in the treatment of opiate overdose. The same drug, when swallowed, acts exclusively on the bowels; it is here used to treat constipation under opiate pain therapy and does not affect the pain-reducing effect of the opiate.
Oral[edit]
The oral route is generally the most convenient and costs the least.[25]However, some drugs can causegastrointestinal tractirritation.[26]For drugs that come indelayed releaseortime-releaseformulations, breaking the tablets or capsules can lead to more rapid delivery of the drug than intended.[25]The oral route is limited toformulationscontainingsmall moleculesonly while biopharmaceuticals (usually proteins) would be digested in the stomach and thereby become ineffective. Biopharmaceuticals have to be given by injection or infusion. However, recent research found various ways to improve oral bioavailability of these drugs. In particular permeation enhancers,[27]ionic liquids,[28]lipid-based nanocarriers,[29]enzyme inhibitors and microneedles[30]have shown potential.
Oral administrationis often denoted "PO" from "per os", the Latin for "by mouth".
The bioavailability of oral administration is affected by the amount of drug that is absorbed across theintestinal epitheliumandfirst-pass metabolism.[31]
Oral mucosal[edit]
Theoral mucosais the mucous membrane lining the inside of themouth.
Buccal[edit]
Buccally administeredmedication is achieved by placing the drug betweengumsand the inner lining of thecheek.[32][33]In comparison with sublingual tissue, buccal tissue is less permeable resulting in slowerabsorption.[33]
Sublabial[edit]
Sublingual[edit]
Sublingual administrationis fulfilled by placing the drug between the tongue and the lower surface of the mouth.[33]Thesublingual mucosais highly permeable and thereby provides access to the underlying expansive network composed of capillaries, leading to rapid drug absorption.[33]
Intranasal[edit]
Drug administration via the nasal cavity yields rapid drug absorption and therapeutic effects.[33]This is because drug absorption through the nasal passages does not go through the gut before enteringcapillariessituated attissue cellsand then systemic circulation and such absorption route allows transport of drugs into thecentral nervous systemvia the pathways ofolfactoryandtrigeminal nerve.[33]
Intranasal absorption features low lipophilicity, enzymatic degradation within the nasal cavity, large molecular size, and rapid mucociliary clearance from the nasal passages, which explains the lowrisk of systemic exposure of the administered drug absorbedvia intranasal.[33]

Local[edit]
By delivering drugs almost directly to the site of action, the risk of systemicside effectsis reduced.[25]
Skin absorption (dermal absorption), for example, is to directly deliver drug to the skin and, hopefully, to the systemic circulation.[34]However, skin irritation may result, and for some forms such as creams or lotions, the dosage is difficult to control.[26]Upon contact with the skin, the drug penetrates into the deadstratum corneumand can afterwards reach the viableepidermis,thedermis,and theblood vessels.[34]
Parenteral[edit]
The term parenteral is from para-1 'beside' + Greek enteron 'intestine' + -al. This name is due to the fact that it encompasses a route of administration that is not intestinal. However, in common English the term has mostly been used to describe the four most well-known routes of injection.


The term injection encompassesintravenous(IV),intramuscular(IM),subcutaneous(SC) andintradermal(ID) administration.[35]
Parenteral administration generally acts more rapidly than topical or enteral administration, with onset of action often occurring in 15–30 seconds for IV, 10–20 minutes for IM and 15–30 minutes for SC.[36]They also have essentially 100%bioavailabilityand can be used for drugs that are poorly absorbed or ineffective when they are given orally.[25]Some medications, such as certainantipsychotics,can be administered aslong-acting intramuscular injections.[37]OngoingIV infusionscan be used to deliver continuous medication orfluids.[38]
Disadvantages of injections include potential pain or discomfort for the patient and the requirement of trained staff usingaseptic techniquesfor administration.[25]However, in some cases, patients are taught to self-inject, such as SC injection of insulin in patients withinsulin-dependent diabetes mellitus.As the drug is delivered to the site of action extremely rapidly with IV injection, there is a risk ofoverdoseif the dose has been calculated incorrectly, and there is an increased risk of side effects if the drug is administered too rapidly.[25]
Respiratory tract[edit]
Mouth inhalation[edit]

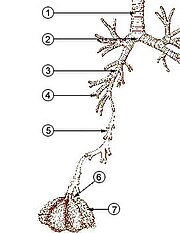
- trachea(conducting zone)
- mainbronchus(conducting zone)
- lobar bronchus(conducting zone)
- segmental bronchus(conducting zone)
- subsegmental bronchus(conducting zone)
- conductingbronchiole(conducting zone)
- terminal bronchiole(conducting zone)
- respiratory bronchiole(transitional respiratory zone)
- alveolar duct(transitional respiratory zone)
- alveolar sac(transitional respiratory zone)
- alveolus(transitional respiratory zone)
Inhaled medications can be absorbed quickly and act both locally and systemically.[26]Proper technique withinhalerdevices is necessary to achieve the correct dose. Some medications can have an unpleasant taste or irritate the mouth.[26]
In general, only 20–50% of the pulmonary-delivered dose rendered in powdery particles will be deposited in the lung upon mouth inhalation.[47]The remainder of 50-70% undeposited aerosolized particles are cleared out of lung as soon asexhalation.[47]
An inhaled powdery particle that is >8 μm is structurally predisposed to depositing in the central and conducting airways (conducting zone) by inertial impaction.[47]
An inhaled powdery particle that is between 3 and 8 μm in diameter tend to largely deposit in thetransitional zonesof the lung by sedimentation.[47]
An inhaled powdery particle that is <3 μm in diameter is structurally predisposed to depositing primarily in therespiratory regionsof the peripheral lung via diffusion.[47]
Particles that deposit in the upper and central airways are generally absorbed systemically to great extent because they are only partially removed bymucociliaryclearance, which results in orally mediated absorption when the transported mucous is swallowed, and first pass metabolism or incomplete absorption through loss at the fecal route can sometimes reduce the bioavailability.[48]This should in no way suggest to clinicians or researchers that inhaled particles are not a greater threat than swallowed particles, it merely signifies that a combination of both methods may occur with some particles, no matter the size of or lipo/hydrophilicity of the different particle surfaces.[47]
Nasal inhalation[edit]
Inhalation by nose of a substance is almost identical to oral inhalation, except that some of the drug is absorbed intranasally instead of in the oral cavity before entering the airways. Both methods can result in varying levels of the substance to be deposited in their respective initial cavities, and the level of mucous in either of these cavities will reflect the amount of substance swallowed. The rate of inhalation will usually determine the amount of the substance which enters the lungs. Faster inhalation results in more rapid absorption because more substance finds the lungs. Substances in a form that resists absorption in the lung will likely resist absorption in the nasal passage, and the oral cavity, and are often even more resistant to absorption after they fail absorption in the former cavities and are swallowed.
Research[edit]
Neural drug delivery is the next step beyond the basic addition ofgrowth factorstonerve guidance conduits.Drug delivery systemsallow the rate of growth factor release to be regulated over time, which is critical for creating an environment more closely representative of in vivo development environments.[49]
See also[edit]
- ADME
- Catheter
- Dosage form
- Drug injection
- Ear instillation
- Hypodermic needle
- Intravenous marijuana syndrome
- List of medical inhalants
- Nanomedicine
- Absorption (pharmacology)
References[edit]
- ^TheFreeDictionary.com > route of administrationArchived2011-06-12 at theWayback MachineCiting: Jonas: Mosby's Dictionary of Complementary and Alternative Medicine. 2005, Elsevier.
- ^Lees P, Cunningham FM, Elliott J (2004)."Principles of pharmacodynamics and their applications in veterinary pharmacology".J. Vet. Pharmacol. Ther.27(6): 397–414.doi:10.1111/j.1365-2885.2004.00620.x.PMID15601436.Archivedfrom the original on 2022-08-18.Retrieved2023-12-09.
- ^abc"topical".Merriam-Websterdictionary.Archivedfrom the original on 2017-07-30.Retrieved2017-07-30.
- ^abthefreedictionary.com > topicalArchived2010-12-05 at theWayback MachineCiting: The American Heritage Dictionary of the English Language, Fourth Edition, 2000
- ^ab"topical".dictionary.com.Archivedfrom the original on 2017-07-30.Retrieved2017-07-30.
- ^abc"Oklahoma Administrative Code and Register > 195:20-1-3.1. Pediatric conscious sedation utilizing enteral methods (oral, rectal, sublingual)".Archivedfrom the original on 2011-07-22.Retrieved2009-01-18.
- ^Davis MP, Walsh D, LeGrand SB, Naughton M (2002). "Symptom control in cancer patients: the clinical pharmacology and therapeutic role of suppositories and rectal suspensions".Support Care Cancer.10(2): 117–38.doi:10.1007/s00520-001-0311-6.PMID11862502.S2CID30569818.
- ^De Boer AG, Moolenaar F, de Leede LG, Breimer DD (1982). "Rectal drug administration: clinical pharmacokinetic considerations".Clin Pharmacokinetics.7(4): 285–311.doi:10.2165/00003088-198207040-00002.PMID6126289.S2CID41562861.
- ^Van Hoogdalem EJ, de Boer AG, Breimer DD (1991). "Pharmacokinetics of rectal drug administration, Part 1".Clin Pharmacokinet.21(1): 11–26.doi:10.2165/00003088-199121010-00002.PMID1717195.S2CID35104089.
- ^Van Hoogdalem EJ, de Boer AG, Breimer DD (1991). "Pharmacokinetics of rectal drug administration, Part 2".Clin Pharmacokinet.21(2): 110–128.doi:10.2165/00003088-199121020-00003.PMID1884566.S2CID11720029.
- ^Moolenaar F, Koning B, Huizinga T (1979). "Biopharmaceutics of rectal administration of drugs in man. Absorption rate and bioavailability of phenobarbital and its sodium salt from rectal dosage forms".International Journal of Pharmaceutics.4(2): 99–109.doi:10.1016/0378-5173(79)90057-7.
- ^Graves NM, Holmes GB, Kriel RL, Jones-Saete C, Ong B, Ehresman DJ (1989). "Relative bioavailability of rectally administered phenobarbital sodium parenteral solution".DICP: The Annals of Pharmacotherapy.23(7–8): 565–568.doi:10.1177/1060028089023007-806.PMID2763578.S2CID27397387.
- ^Moolenaar S, Bakker S, Visser J, Huizinga T (1980). "Biopharmaceutics of rectal administration of drugs in man IX. Comparative biopharmaceutics of diazepam after single rectal, oral, intramuscular and intravenous administration in man".International Journal of Pharmaceutics.5(2): 127–137.doi:10.1016/0378-5173(80)90017-4.
- ^[1]Archived2013-01-26 at theWayback MachineNee, Douglas, Pharm D, MS. "Rectal Administration of Medications at the End of Life". HPNA Teleconference, December 6, 2006, accessed November 2013.
- ^"Use of Rectal Meds for Palliative Care Patients. End of Life / Palliative Education Resource Center, Medical College of Wisconsin".mcw.edu.Archived fromthe originalon 2 June 2014.Retrieved14 April2018.
- ^"injection".Cambridge dictionary.Archivedfrom the original on 2017-07-30.Retrieved2017-07-30.
- ^"MDMA (ecstasy) metabolites and neurotoxicity: No occurrence of MDMA neurotoxicity from metabolites when injected directly into brain, study shows".Neurotransmitter.net.Archivedfrom the original on 2010-08-07.Retrieved2010-08-19.
- ^McKeran RO, Firth G, Oliver S, Uttley D, O'Laoire S; Firth, G; Oliver, S; Uttley, D; O'Laoire, S (2010-07-06)."A potential application for the intracerebral injection of drugs entrapped within liposomes in the treatment of human cerebral gliomas".Journal of Neurology, Neurosurgery, and Psychiatry.48(12): 1213–1219.doi:10.1136/jnnp.48.12.1213.PMC1028604.PMID2418156.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Wright JL, Merchant RE (1994). "Blood–brain barrier changes following intracerebral injection of human recombinant tumor necrosis factor-α in the rat".Journal of Neuro-Oncology.20(1): 17–25.doi:10.1007/BF01057957.PMID7807180.S2CID12445653.
- ^"Acute Decreases in Cerebrospinal Fluid Glutathione Levels after Intracerebroventricular Morphine for Cancer Pain".Anesthesia-analgesia.org. 1999-06-22.Archivedfrom the original on 2006-01-06.Retrieved2010-08-19.
- ^"Crystal Meth: The Effects".Fenway Community Health.Fenway Health.Archived fromthe originalon 10 December 2010.
- ^Malenka, Eric J. Nestler, Steven E. Hyman, Robert C. (2009).Molecular Neuropharmacology: A Foundation for Clinical Neuroscience(2nd ed.). New York: McGraw-Hill Medical.ISBN978-0-07-148127-4.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Mylonaki I, Allémann É, Saucy F, Haefliger J-A, Delie F, Jordan O (2017)."Perivascular medical devices and drug delivery systems: Making the right choices".Biomaterials.128:56–68.doi:10.1016/j.biomaterials.2017.02.028.PMID28288349.Archivedfrom the original on 2020-04-23.Retrieved2020-03-23.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^"Vancocin".The American Society of Health-System Pharmacists.Archivedfrom the original on September 6, 2015.RetrievedSep 4,2015.
- ^abcdef"The Administration of Medicines".Nursing Practice Clinical Zones: Prescribing.NursingTimes.net. 2007.Archivedfrom the original on January 2, 2020.RetrievedApril 2,2013.
- ^abcd"DDS Medication Administration Recertification Manual"(PDF).DDS Recertification Review Manual.State ofConnecticut Department of Developmental Services.2006.Archived(PDF)from the original on May 14, 2013.RetrievedApril 2,2013.
- ^Yamamoto, A; Ukai, H; Morishita, M; Katsumi, H (2020). "Approaches to improve intestinal and transmucosal absorption of peptide and protein drugs".Adv Drug Deliv Rev.211:107537.doi:10.1016/j.pharmthera.2020.107537.PMID32201316.S2CID214618193.
- ^Banerjee, Amrita; Ibsen, Kelly; Brown, Tyler; Chen, Renwei; Agatemor, Christian; Mitragotri, Samir (2018-06-20)."Ionic liquids for oral insulin delivery".Proceedings of the National Academy of Sciences.115(28): 7296–7301.Bibcode:2018PNAS..115.7296B.doi:10.1073/pnas.1722338115.ISSN0027-8424.PMC6048483.PMID29941553.
- ^Haddadzadegan, S; Dorkoosh, F; Bernkop-Schnürch, A (2022)."Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers".Adv Drug Deliv Rev.182:114097.doi:10.1016/j.addr.2021.114097.PMID34999121.
- ^Maher, S; Brayden, DJ (2021)."Approaches to improve intestinal and transmucosal absorption of peptide and protein drugs".Pharmacol Ther.177:113925.doi:10.1016/j.addr.2021.113925.PMID34418495.
- ^Hebert, Mary F. (2013). "Impact of Pregnancy on Maternal Pharmacokinetics of Medications".Clinical Pharmacology During Pregnancy.Elsevier. pp. 17–39.doi:10.1016/b978-0-12-386007-1.00003-9.ISBN978-0-12-386007-1.
- ^LE.JENNIFER (2020-03-27)."Drug Absorption - Clinical Pharmacology".MSD Manual Professional Edition.Archivedfrom the original on 2019-10-31.Retrieved2020-03-28.
- ^abcdefgKaminsky, Bonnie M.; Bostwick, Jolene R.; Guthrie, Sally K. (2015-04-23). "Alternate Routes of Administration of Antidepressant and Antipsychotic Medications".The Annals of Pharmacotherapy.49(7). SAGE Publications: 808–817.doi:10.1177/1060028015583893.ISSN1060-0280.PMID25907529.S2CID39802797.
- ^abRodrigues, Francisca; Oliveira, Maria Beatriz P.P. (2016). "Cell-based in vitro models for dermal permeability studies".Concepts and Models for Drug Permeability Studies.Elsevier. pp. 155–167.doi:10.1016/b978-0-08-100094-6.00010-9.ISBN978-0-08-100094-6.
- ^"List of Error-Prone Abbreviations"(PDF).Institute For Safe Medication Practices.Archived(PDF)from the original on 22 February 2016.Retrieved14 April2018.
- ^"Routes for Drug Administration"(PDF).Emergency Treatment Guidelines Appendix.Manitoba Health. 2003. Archived fromthe original(PDF)on October 1, 2013.RetrievedApril 2,2013.
- ^Stahl SM, Stahl's Essential Psychopharmacology: Neuroscientific basis and practical applications, New York: Cambridge University Press, 2008
- ^Smeltzer SC Bare BG, Textbook of Medical-Surgical Nursing, 9th ed, Philadelphia: Lippincott, 2000.
- ^Ali, Mohammed (2010). "Pulmonary Drug Delivery".Handbook of Non-Invasive Drug Delivery Systems.Elsevier. pp. 209–246.doi:10.1016/b978-0-8155-2025-2.10009-5.ISBN978-0-8155-2025-2.
- ^"Lungs - Anatomy of the Respiratory System".archive.is.2020-03-29. Archived fromthe originalon 2020-03-29.Retrieved2020-03-29.
- ^"Major Zones & Divisions".GetBodySmart.2017-10-30.Retrieved2020-03-29.
- ^"Diagram for conducting zone".Archivedfrom the original on 2020-03-29.Retrieved2020-03-29.
- ^"Diagram for respiratory zone".Archivedfrom the original on 2020-03-29.Retrieved2020-03-29.
- ^"Diagram for upper respiratory tract".Archivedfrom the original on 2020-03-29.Retrieved2020-03-29.
- ^"Diagram for lower respiratory tract".Archivedfrom the original on 2020-03-29.Retrieved2020-03-29.
- ^"Diagram for majour zones of respiratory system".Archivedfrom the original on 2020-03-29.Retrieved2020-03-29.
- ^abcdefTandel, Hemal; Florence, Kiruba; Misra, Ambikanandan (2011). "Protein and Peptide Delivery through Respiratory Pathway".Challenges in Delivery of Therapeutic Genomics and Proteomics.Elsevier. pp. 429–479.doi:10.1016/b978-0-12-384964-9.00009-8.ISBN978-0-12-384964-9.
- ^Bustamante-Marin, Ximena M.; Ostrowski, Lawrence E. (April 18, 2017)."Cilia and Mucociliary Clearance".Cold Spring Harbor Perspectives in Biology.9(4): a028241.doi:10.1101/cshperspect.a028241.PMC5378048.PMID27864314.
- ^Lavik, E. and R. Langer, Tissue engineering; current state and perspectives. Applied Microbiology Biotechnology, 2004. 65: p. 1–8
External links[edit]
- The 10th US-Japan Symposium on Drug Delivery Systems
- FDA Center for Drug Evaluation and Research Data Standards Manual: Route of Administration.
- FDA Center for Drug Evaluation and Research Data Standards Manual: Dosage Form.
- A.S.P.E.N. American Society for Parenteral and Enteral Nutrition
- As Drug+Administration+Routesat the U.S. National Library of MedicineMedical Subject Headings(MeSH)






