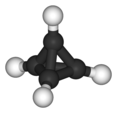
Alkane
Inorganic chemistry,analkane,orparaffin(a historicaltrivial namethat also hasother meanings), is anacyclicsaturatedhydrocarbon.In other words, an alkane consists ofhydrogenandcarbonatoms arranged in atreestructure in which all thecarbon–carbon bondsaresingle.[1]Alkanes have the general chemical formulaCnH2n+2.The alkanes range in complexity from the simplest case ofmethane(CH4), wheren= 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, likepentacontane(C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, anisomeroftetradecane(C14H30).
TheInternational Union of Pure and Applied Chemistry(IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formulaCnH2n+2,and therefore consisting entirely of hydrogen atoms and saturated carbon atoms ". However, some sources use the term to denoteanysaturated hydrocarbon, including those that are either monocyclic (i.e. thecycloalkanes) orpolycyclic,despite them having a distinct general formula (e.g. cycloalkanes areCnH2n).
In an alkane, each carbon atom issp3-hybridizedwith 4sigma bonds(either C–C orC–H), and each hydrogen atom is joined to one of the carbon atoms (in a C–H bond). The longest series of linked carbon atoms in a molecule is known as itscarbon skeletonor carbon backbone. The number of carbon atoms may be considered as the size of the alkane.
One group of thehigher alkanesarewaxes,solids atstandard ambient temperature and pressure(SATP), for which the number of carbon atoms in the carbon backbone is greater than about 17. With their repeated –CH2units, the alkanes constitute ahomologous seriesof organic compounds in which the members differ inmolecular massby multiples of 14.03u(the total mass of each suchmethylene-bridgeunit, which comprises a single carbon atom of mass 12.01 u and two hydrogen atoms of mass ~1.01 u each).
Methane is produced bymethanogenic bacteriaand some long-chain alkanes function as pheromones in certain animal species or as protective waxes in plants and fungi. Nevertheless, most alkanes do not have muchbiological activity.They can be viewed as molecular trees upon which can be hung the more active/reactivefunctional groupsof biological molecules.
The alkanes have two main commercial sources:petroleum(crude oil) andnatural gas.
Analkylgroup is an alkane-based molecular fragment that bears one open valence for bonding. They are generally abbreviated with the symbol for anyorganyl group,R, although Alk is sometimes used to specifically symbolize an alkyl group (as opposed to an alkenyl group or aryl group).
Structure and classification
[edit]Ordinarily the C-C single bond distance is 1.53ångströms(1.53×10−10m).[2] Saturated hydrocarbons can be linear, branched, orcyclic.The third group is sometimes calledcycloalkanes.[1]Very complicated structures are possible by combining linear, branch, cyclic alkanes.
Isomerism
[edit]
Alkanes with more than threecarbonatoms can be arranged in various ways, formingstructural isomers.The simplest isomer of an alkane is the one in which the carbon atoms are arranged in a single chain with no branches. This isomer is sometimes called then-isomer (nfor "normal", although it is not necessarily the most common). However, the chain of carbon atoms may also be branched at one or more points. The number of possible isomers increases rapidly with the number of carbon atoms. For example, for acyclic alkanes:[3]
- C1:methaneonly
- C2:ethaneonly
- C3:propaneonly
- C4:2 isomers:butaneandisobutane
- C5:3 isomers:pentane,isopentane,andneopentane
- C6:5 isomers:hexane,2-methylpentane,3-methylpentane,2,2-dimethylbutane,and2,3-dimethylbutane
- C7:9 isomers:heptane,2-methylhexane,3-methylhexane,2,2-dimethylpentane,2,3-dimethylpentane,2,4-dimethylpentane,3,3-dimethylpentane,3-ethylpentane,2,2,3-trimethylbutane
- C8:18 isomers:octane,2-methylheptane,3-methylheptane,4-methylheptane,2,2-dimethylhexane,2,3-dimethylhexane,2,4-dimethylhexane,2,5-dimethylhexane,3,3-dimethylhexane,3,4-dimethylhexane,3-ethylhexane,2,2,3-trimethylpentane,2,2,4-trimethylpentane,2,3,3-trimethylpentane,2,3,4-trimethylpentane,3-ethyl-2-methylpentane,3-ethyl-3-methylpentane,2,2,3,3-tetramethylbutane
- C9:35 isomers
- C10:75 isomers
- C12:355 isomers
- C32:27,711,253,769 isomers
- C60:22,158,734,535,770,411,074,184 isomers, many of which are not stable
Branched alkanes can bechiral.For example,3-methylhexaneand its higherhomologuesare chiral due to theirstereogenic centerat carbon atom number 3. The above list only includes differences of connectivity, not stereochemistry. In addition to the alkane isomers, the chain of carbon atoms may form one or more rings. Such compounds are calledcycloalkanes,and are also excluded from the above list because changing the number of rings changes themolecular formula.For example,cyclobutaneandmethylcyclopropaneare isomers of each other (C4H8), but are not isomers of butane (C4H10).
Branched alkanes are more thermodynamically stable than their linear (or less branched) isomers. For example, the highly branched 2,2,3,3-tetramethylbutane is about 1.9 kcal/mol more stable than its linear isomer,n-octane.[4]
Nomenclature
[edit]TheIUPAC nomenclature(systematic way of naming compounds) for alkanes is based on identifying hydrocarbon chains. Unbranched, saturated hydrocarbon chains are named systematically with a Greek numerical prefix denoting the number of carbons and the suffix "-ane".[5]
In 1866,August Wilhelm von Hofmannsuggested systematizing nomenclature by using the whole sequence of vowels a, e, i, o and u to create suffixes -ane, -ene, -ine (or -yne), -one, -une, for thehydrocarbonsCnH2n+2,CnH2n,CnH2n−2,CnH2n−4,CnH2n−6.[6]In modern nomenclature, the first three specifically name hydrocarbons with single, double and triple bonds;[7]while "-one" now represents aketone.
Linear alkanes
[edit]Straight-chain alkanes are sometimes indicated by the prefix "n-" or "n- "(for" normal ") where a non-linearisomerexists. Although this is not strictly necessary and is not part of the IUPAC naming system, the usage is still common in cases where one wishes to emphasize or distinguish between the straight-chain and branched-chain isomers, e.g., "n-butane"rather than simply" butane "to differentiate it fromisobutane.Alternative names for this group used in the petroleum industry arelinear paraffinsorn-paraffins.
The first eight members of the series (in terms of number of carbon atoms) are named as follows:
- methane
- CH4– one carbon and 4 hydrogen
- ethane
- C2H6– two carbon and 6 hydrogen
- propane
- C3H8– three carbon and 8 hydrogen
- butane
- C4H10– four carbon and 10 hydrogen
- pentane
- C5H12– five carbon and 12 hydrogen
- hexane
- C6H14– six carbon and 14 hydrogen
- heptane
- C7H16– seven carbons and 16 hydrogen
- octane
- C8H18– eight carbons and 18 hydrogen
The first four names werederivedfrommethanol,ether,propionic acidandbutyric acid.Alkanes with five or more carbon atoms are named by adding thesuffix-aneto the appropriatenumerical multiplierprefix[8]withelisionof any terminal vowel (-aor-o) from the basic numerical term. Hence,pentane,C5H12;hexane,C6H14;heptane,C7H16;octane,C8H18;etc. Thenumeral prefixis generally Greek; however, alkanes with a carbon atom count ending in nine, for examplenonane,use theLatinprefixnon-.
Branched alkanes
[edit]
Simple branched alkanes often have a common name using a prefix to distinguish them from linear alkanes, for examplen-pentane,isopentane,andneopentane.
IUPAC naming conventions can be used to produce a systematic name.
The key steps in the naming of more complicated branched alkanes are as follows:[9]
- Identify the longest continuous chain of carbon atoms
- Name this longest root chain using standard naming rules
- Name each side chain by changing the suffix of the name of the alkane from "-ane" to "-yl"
- Number the longest continuous chain in order to give the lowest possible numbers for the side-chains[10]
- Number and name the side chains before the name of the root chain
- If there are multiple side chains of the same type, use prefixes such as "di-" and "tri-" to indicate it as such, and number each one.
- Add side chain names in alphabetical (disregarding "di-" etc. prefixes) order in front of the name of the root chain
| Common name | n-pentane | isopentane | neopentane |
|---|---|---|---|
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Structure |  |

|
Saturated cyclic hydrocarbons
[edit]Though technically distinct from the alkanes, this class of hydrocarbons is referred to by some as the "cyclic alkanes." As their description implies, they contain one or more rings.
Simple cycloalkanes have a prefix "cyclo-" to distinguish them from alkanes. Cycloalkanes are named as per their acyclic counterparts with respect to the number of carbon atoms in their backbones, e.g.,cyclopentane(C5H10) is a cycloalkane with 5 carbon atoms just likepentane(C5H12), but they are joined up in a five-membered ring. In a similar manner,propaneandcyclopropane,butaneandcyclobutane,etc.
Substituted cycloalkanes are named similarly to substituted alkanes – the cycloalkane ring is stated, and the substituents are according to their position on the ring, with the numbering decided by theCahn–Ingold–Prelog priority rules.[8]
Trivial/common names
[edit]The trivial (non-systematic) name for alkanes is 'paraffins'. Together, alkanes are known as the 'paraffin series'. Trivial names for compounds are usually historical artifacts. They were coined before the development of systematic names, and have been retained due to familiar usage in industry. Cycloalkanes are also called naphthenes.[11][12]
Branched-chain alkanes are calledisoparaffins."Paraffin" is a general term and often does not distinguish between pure compounds and mixtures ofisomers,i.e., compounds of the samechemical formula,e.g.,pentaneandisopentane.
- In IUPAC
The following trivial names are retained in the IUPAC system:
- isobutanefor 2-methylpropane
- isopentanefor 2-methylbutane
- neopentanefor 2,2-dimethylpropane.
- Non-IUPAC
Some non-IUPAC trivial names are occasionally used:
- cetane, forhexadecane
- cerane, forhexacosane[13]
Physical properties
[edit]All alkanes are colorless.[14][15]Alkanes with the lowest molecular weights are gases, those of intermediate molecular weight are liquids, and the heaviest are waxy solids.[16][17]
Table of alkanes
[edit]| Alkane | Formula | Boiling point[note 1] [°C] |
Melting point[note 1] [°C] |
Density[note 1] [kg/m3] (at 20 °C) |
Isomers[note 2] |
|---|---|---|---|---|---|
| Methane | CH4 | −162 | −182 | 0.656 (gas) | 1 |
| Ethane | C2H6 | −89 | −183 | 1.26 (gas) | 1 |
| Propane | C3H8 | −42 | −188 | 2.01 (gas) | 1 |
| Butane | C4H10 | 0 | −138 | 2.48 (gas) | 2 |
| Pentane | C5H12 | 36 | −130 | 626 (liquid) | 3 |
| Hexane | C6H14 | 69 | −95 | 659 (liquid) | 5 |
| Heptane | C7H16 | 98 | −91 | 684 (liquid) | 9 |
| Octane | C8H18 | 126 | −57 | 703 (liquid) | 18 |
| Nonane | C9H20 | 151 | −54 | 718 (liquid) | 35 |
| Decane | C10H22 | 174 | −30 | 730 (liquid) | 75 |
| Undecane | C11H24 | 196 | −26 | 740 (liquid) | 159 |
| Dodecane | C12H26 | 216 | −10 | 749 (liquid) | 355 |
| Tridecane | C13H28 | 235 | −5.4 | 756 (liquid) | 802 |
| Tetradecane | C14H30 | 253 | 5.9 | 763 (liquid) | 1858 |
| Pentadecane | C15H32 | 270 | 10 | 769 (liquid) | 4347 |
| Hexadecane | C16H34 | 287 | 18 | 773 (liquid) | 10,359 |
| Heptadecane | C17H36 | 303 | 22 | 777 (solid) | 24,894 |
| Octadecane | C18H38 | 317 | 28 | 781 (solid) | 60,523 |
| Nonadecane | C19H40 | 330 | 32 | 785 (solid) | 148,284 |
| Icosane | C20H42 | 343 | 37 | 789 (solid) | 366,319 |
| Triacontane | C30H62 | 450 | 66 | 810 (solid) | 4,111,846,763 |
| Tetracontane | C40H82 | 525 | 82 | 817 (solid) | 62,481,801,147,341 |
| Pentacontane | C50H102 | 575 | 91 | 824 (solid) | 1,117,743,651,746,953,270 |
| Hexacontane | C60H122 | 625 | 100 | 829 (solid) | 2.21587345357704×1022 |
| Heptacontane | C70H142 | 653 | 109 | 869 (solid) | 4.71484798515330×1026 |
| |||||
Boiling point
[edit]
Alkanes experience intermolecularvan der Waals forces.The cumulative effects of these intermolecular forces give rise to greater boiling points of alkanes.[18]
Two factors influence the strength of the van der Waals forces:
- the number of electrons surrounding themolecule,which increases with the alkane's molecular weight
- the surface area of the molecule
Understandard conditions,from CH4to C4H10alkanes are gaseous; from C5H12to C17H36they are liquids; and after C18H38they are solids. As the boiling point of alkanes is primarily determined by weight, it should not be a surprise that the boiling point has an almost linear relationship with the size (molecular weight) of the molecule. As a rule of thumb, the boiling point rises 20–30 °C for each carbon added to the chain; this rule applies to other homologous series.[18]
A straight-chain alkane will have a boiling point higher than a branched-chain alkane due to the greater surface area in contact, and thus greater van der Waals forces, between adjacent molecules. For example, compareisobutane(2-methylpropane) andn-butane(butane), which boil at −12 and 0 °C, and 2,2-dimethylbutane and 2,3-dimethylbutane which boil at 50 and 58 °C, respectively.[18]
On the other hand, cycloalkanes tend to have higher boiling points than their linear counterparts due to the locked conformations of the molecules, which give a plane of intermolecular contact.
Melting points
[edit]Themelting pointsof the alkanes follow a similar trend toboiling pointsfor the same reason as outlined above. That is, (all other things being equal) the larger the molecule the higher the melting point. There is one significant difference between boiling points and melting points. Solids have a more rigid and fixed structure than liquids. This rigid structure requires energy to break down. Thus the better put together solid structures will require more energy to break apart. For alkanes, this can be seen from the graph above (i.e., the blue line). The odd-numbered alkanes have a lower trend in melting points than even-numbered alkanes. This is because even-numbered alkanes pack well in the solid phase, forming a well-organized structure which requires more energy to break apart. The odd-numbered alkanes pack less well and so the "looser" -organized solid packing structure requires less energy to break apart.[19]For a visualization of the crystal structures see.[20]
The melting points of branched-chain alkanes can be either higher or lower than those of the corresponding straight-chain alkanes, again depending on the ability of the alkane in question to pack well in the solid phase.
Conductivity and solubility
[edit]Alkanes do not conduct electricity in any way, nor are they substantiallypolarizedby anelectric field.For this reason, they do not formhydrogen bondsand are insoluble in polar solvents such as water. Since the hydrogen bonds between individual water molecules are aligned away from an alkane molecule, the coexistence of an alkane and water leads to an increase in molecular order (a reduction inentropy). As there is no significant bonding between water molecules and alkane molecules, thesecond law of thermodynamicssuggests that this reduction in entropy should be minimized by minimizing the contact between alkane and water: Alkanes are said to behydrophobicas they are insoluble in water.
Their solubility in nonpolar solvents is relatively high, a property that is calledlipophilicity.Alkanes are, for example, miscible in all proportions among themselves.
The density of the alkanes usually increases with the number of carbon atoms but remains less than that of water. Hence, alkanes form the upper layer in an alkane–water mixture.[21]
Molecular geometry
[edit]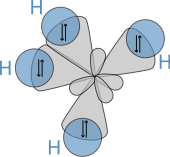
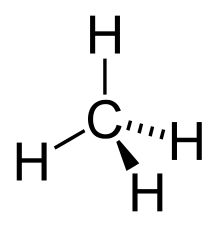
The molecular structure of the alkanes directly affects their physical and chemical characteristics. It is derived from theelectron configurationofcarbon,which has fourvalence electrons.The carbon atoms in alkanes are described as sp3hybrids; that is to say that, to a good approximation, the valence electrons are in orbitals directed towards the corners of a tetrahedron which are derived from the combination of the 2s orbital and the three 2p orbitals. Geometrically, the angle between the bonds are cos−1(−1/3) ≈ 109.47°. This is exact for the case of methane, while larger alkanes containing a combination of C–H and C–C bonds generally have bonds that are within several degrees of this idealized value.
Bond lengths and bond angles
[edit]
An alkane has only C–H and C–C single bonds. The former result from the overlap of an sp3orbital of carbon with the 1s orbital of a hydrogen; the latter by the overlap of two sp3orbitals on adjacent carbon atoms. Thebond lengthsamount to 1.09 × 10−10m for a C–H bond and 1.54 × 10−10m for a C–C bond.
The spatial arrangement of the bonds is similar to that of the four sp3orbitals—they are tetrahedrally arranged, with an angle of 109.47° between them. Structural formulae that represent the bonds as being at right angles to one another, while both common and useful, do not accurately depict the geometry.
Conformation
[edit]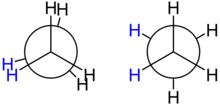
The spatial arrangement of the C-C and C-H bonds are described by the torsion angles of the molecule is known as itsconformation.Inethane,the simplest case for studying the conformation of alkanes, there is nearly free rotation about a carbon–carbon single bond. Two limiting conformations are important:eclipsedconformation andstaggered conformation.The staggered conformation is 12.6 kJ/mol (3.0 kcal/mol) lower in energy (more stable) than the eclipsed conformation (the least stable). In highly branched alkanes, the bond angle may differ from the optimal value (109.5°) to accommodate bulky groups. Such distortions introduce a tension in the molecule, known assteric hindranceor strain. Strain substantially increases reactivity.[22]
Spectroscopic properties
[edit]Spectroscopic signatures for alkanes are obtainable by the major characterization techniques.[23]
Infrared spectroscopy
[edit]The C-H stretching mode gives a strong absorptions between 2850 and 2960cm−1and weaker bands for the C-C stretching mode absorbs between 800 and 1300 cm−1.The carbon–hydrogen bending modes depend on the nature of the group: methyl groups show bands at 1450 cm−1and 1375 cm−1,while methylene groups show bands at 1465 cm−1and 1450 cm−1.[24]Carbon chains with more than four carbon atoms show a weak absorption at around 725 cm−1.
NMR spectroscopy
[edit]The proton resonances of alkanes are usually found atδH= 0.5–1.5. The carbon-13 resonances depend on the number of hydrogen atoms attached to the carbon:δC= 8–30 (primary, methyl, –CH3), 15–55 (secondary, methylene, –CH2–), 20–60 (tertiary, methyne, C–H) and quaternary. The carbon-13 resonance of quaternary carbon atoms is characteristically weak, due to the lack ofnuclear Overhauser effectand the longrelaxation time,and can be missed in weak samples, or samples that have not been run for a sufficiently long time.
Mass spectrometry
[edit]Since alkanes have highionization energies,theirelectron impact mass spectrashow weak currents for their molecular ions. The fragmentation pattern can be difficult to interpret, but in the case of branched chain alkanes, the carbon chain is preferentially cleaved at tertiary or quaternary carbons due to the relative stability of the resultingfree radicals.The mass spectra for straight-chain alkanes is illustrated by that fordodecane:the fragment resulting from the loss of a single methyl group (M− 15) is absent, fragments are more intense than the molecular ion and are spaced by intervals of 14 mass units, corresponding to loss of CH2groups.[25]
Chemical properties
[edit]Alkanes are only weakly reactive with most chemical compounds. They only reacts with the strongest of electrophilic reagents by virtue of their strong C–H bonds (~100 kcal/mol) and C–C bonds (~90 kcal/mol). They are also relatively unreactive toward free radicals. This inertness is the source of the termparaffins(with the meaning here of "lacking affinity" ). Incrude oilthe alkane molecules have remained chemically unchanged for millions of years.
Acid-base behavior
[edit]Theacid dissociation constant(pKa) values of all alkanes are estimated to range from 50 to 70, depending on the extrapolation method, hence they are extremely weak acids that are practically inert to bases (see:carbon acids). They are also extremely weak bases, undergoing no observable protonation in puresulfuric acid(H0~ −12), althoughsuperacidsthat are at least millions of times stronger have been known to protonate them to give hypercoordinate alkanium ions (see:methanium ion). Thus, a mixture ofantimony pentafluoride(SbF5) andfluorosulfonic acid(HSO3F), calledmagic acid,can protonate alkanes.[26]
Reactions with oxygen (combustion reaction)
[edit]All alkanes react withoxygenin acombustionreaction, although they become increasingly difficult to ignite as the number of carbon atoms increases. The general equation for complete combustion is:
- CnH2n+2+ (3/2n+1/2) O2→ (n+ 1) H2O +nCO2
- or CnH2n+2+ (3n+ 1/2) O2→ (n+ 1) H2O +nCO2
In the absence of sufficient oxygen,carbon monoxideor evensootcan be formed, as shown below:
For example,methane:
- 2 CH4+ 3 O2→ 4 H2O + 2 CO
- CH4+ O2→ 2 H2O + C
See thealkane heat of formation tablefor detailed data. Thestandard enthalpy change of combustion,ΔcH⊖,for alkanes increases by about 650 kJ/mol per CH2group. Branched-chain alkanes have lower values of ΔcH⊖than straight-chain alkanes of the same number of carbon atoms, and so can be seen to be somewhat more stable.
Biodegradation
[edit]Some organisms are capable of metalbolizing alkanes.[27][28]Themethane monooxygenasesconvert methane tomethanol.For higher alkanes,cytochrome P450convert alkanes to alcohols, which are then susceptible to degradation.
Free radical reactions
[edit]Free radicals,molecules with unpaired electrons, play a large role in most reactions of alkanes.Free radical halogenationreactions occur with halogens, leading to the production ofhaloalkanes.The hydrogen atoms of the alkane are progressively replaced by halogen atoms. The reaction of alkanes and fluorine is highlyexothermicand can lead to an explosion.[29]These reactions are an important industrial route to halogenated hydrocarbons. There are three steps:
- Initiationthe halogen radicals form byhomolysis.Usually, energy in the form of heat or light is required.
- Chain reactionorPropagationthen takes place—the halogen radical abstracts a hydrogen from the alkane to give an alkyl radical. This reacts further.
- Chain terminationwhere the radicals recombine.
Experiments have shown that all halogenation produces a mixture of all possible isomers, indicating that all hydrogen atoms are susceptible to reaction. The mixture produced, however, is not statistical: Secondary and tertiary hydrogen atoms are preferentially replaced due to the greater stability of secondary and tertiary free-radicals. An example can be seen in the monobromination of propane:[18]

In theReed reaction,sulfur dioxideandchlorineconvert hydrocarbons tosulfonyl chloridesunder the influence oflight.
Under some conditions, alkanes will undergoNitration.
C-H activation
[edit]Certain transition metal complexes promote non-radical reactions with alkanes, resulting in soC–H bond activationreactions.[30]
Cracking
[edit]Cracking breaks larger molecules into smaller ones. This reaction requires heat and catalysts. The thermal cracking process follows ahomolyticmechanism with formation offree radicals.The catalytic cracking process involves the presence ofacidcatalysts(usually solid acids such assilica-aluminaandzeolites), which promote aheterolytic(asymmetric) breakage of bonds yielding pairs of ions of opposite charges, usually acarbocation.Carbon-localized free radicals and cations are both highly unstable and undergo processes of chain rearrangement, C–C scission in positionbeta(i.e., cracking) andintra-andintermolecularhydrogen transfer orhydridetransfer. In both types of processes, the correspondingreactive intermediates(radicals, ions) are permanently regenerated, and thus they proceed by a self-propagating chain mechanism. The chain of reactions is eventually terminated by radical or ion recombination.[citation needed]
Isomerization and reformation
[edit]Dragan and his colleague were the first to report about isomerization in alkanes.[31]Isomerization and reformation are processes in which straight-chain alkanes are heated in the presence of aplatinumcatalyst. In isomerization, the alkanes become branched-chain isomers. In other words, it does not lose any carbons or hydrogens, keeping the same molecular weight.[31]In reformation, the alkanes becomecycloalkanesoraromatic hydrocarbons,giving off hydrogen as a by-product. Both of these processes raise theoctane numberof the substance. Butane is the most common alkane that is put under the process of isomerization, as it makes many branched alkanes with high octane numbers.[31]
Other reactions
[edit]Insteam reforming,alkanes react withsteamin the presence of anickelcatalystto givehydrogenand carbon monoxide.
Occurrence
[edit]Occurrence of alkanes in the Universe
[edit]

Alkanes form a small portion of theatmospheresof the outer gas planets such asJupiter(0.1% methane, 2ppmethane),Saturn(0.2% methane, 5 ppm ethane),Uranus(1.99% methane, 2.5 ppm ethane) andNeptune(1.5% methane, 1.5 ppm ethane).Titan(1.6% methane), a satellite of Saturn, was examined by theHuygensprobe,which indicated that Titan's atmosphere periodically rains liquid methane onto the moon's surface.[32]Also on Titan, the Cassini mission has imaged seasonal methane/ethane lakes near the polar regions of Titan.Methaneandethanehave also been detected in the tail of thecomet Hyakutake.Chemical analysis showed that the abundances of ethane and methane were roughly equal, which is thought to imply that its ices formed in interstellar space, away from the Sun, which would have evaporated these volatile molecules.[33]Alkanes have also been detected inmeteoritessuch ascarbonaceous chondrites.
Occurrence of alkanes on Earth
[edit]Traces of methane gas (about 0.0002% or 1745 ppb) occur in the Earth's atmosphere, produced primarily bymethanogenicmicroorganisms, such asArchaeain the gut of ruminants.[34]
The most important commercial sources for alkanes are natural gas andoil.[18]Natural gas contains primarily methane and ethane, with somepropaneandbutane:oil is a mixture of liquid alkanes and otherhydrocarbons.These hydrocarbons were formed when marine animals and plants (zooplankton and phytoplankton) died and sank to the bottom of ancient seas and were covered with sediments in ananoxicenvironment and converted over many millions of years at high temperatures and high pressure to their current form. Natural gas resulted thereby for example from the following reaction:
- C6H12O6→ 3 CH4+ 3 CO2
These hydrocarbon deposits, collected in porous rocks trapped beneath impermeable cap rocks, comprise commercialoil fields.They have formed over millions of years and once exhausted cannot be readily replaced. The depletion of these hydrocarbons reserves is the basis for what is known as theenergy crisis.
Alkanes have a low solubility in water, so the content in the oceans is negligible; however, at high pressures and low temperatures (such as at the bottom of the oceans), methane can co-crystallize with water to form a solidmethane clathrate(methane hydrate). Although this cannot be commercially exploited at the present time, the amount of combustible energy of the known methane clathrate fields exceeds the energy content of all the natural gas and oil deposits put together. Methane extracted from methane clathrate is, therefore, a candidate for future fuels.
Biological occurrence
[edit]
Aside from petroleum and natural gas, alkanes occur significantly in nature only as methane, which is produced by somearchaeaby the process ofmethanogenesis.These organisms are found in the gut of termites[35]and cows.[36]Themethaneis produced fromcarbon dioxideor other organic compounds. Energy is released by the oxidation ofhydrogen:
- CO2+ 4 H2→ CH4+ 2 H2O
It is probable that our current deposits of natural gas were formed in a similar way.[37]
RCH2\sCH3}} (R =alkyl)
Another route to alkanes ishydrogenolysis,which entails cleavage of C-heteroatom bonds using hydrogen. In industry, the main substrates are organonitrogen and organosulfur impurities, i.e. the heteroatoms are N and S. The specific processes are calledhydrodenitrificationandhydrodesulfurization:
- R3N + 3 H2→ 3 RH + H3N
- R2S + 2 H2→ 2 RH + H2S
Hydrogenolysis can be applied to the conversion of virtually any functional group into hydrocarbons. Substrates include haloalkanes, alcohols, aldehydes, ketones, carboxylic acids, etc. Both hydrogenolysis and hydrogenation are practiced in refineries. The can be effected by usinglithium aluminium hydride,Clemmenson reductionand other specialized routes.
Coal
[edit]Coal is a more traditional precursor to alkanes. A wide range of technologies have been intensively practiced for centuries.[38]Simply heating coal gives alkanes, leaving behindcoke.Relevant technologies include theBergius processandcoal liquifaction.Partial combustion of coal and related solid organic compounds generatescarbon monoxide,which can be hydrogenated using theFischer–Tropsch process.This technology allows the synthesize liquid hydrocarbons, including alkanes. This method is used to produce substitutes forpetroleum distillates.
Laboratory preparation
[edit]Rarely is there any interest in the synthesis of alkanes, since they are usually commercially available and less valued than virtually any precursor. The best-known method ishydrogenationofalkenes.Many C-X bonds can be converted to C-H bonds usinglithium aluminium hydride,Clemmenson reduction,and other specialized routes.[39]Hydrolysis ofAlkyl Grignard reagentsandalkyl lithium compoundsgives alkanes.[40]
Applications
[edit]Fuels
[edit]The dominant use of alkanes is as fuels.Propaneandbutane,easily liquified gases, are commonly known asliquified petroleum gas(LPG).[41]Frompentanetooctanethe alkanes are highly volatile liquids. They are used as fuels ininternal combustion engines,as they vaporize easily on entry into the combustion chamber without forming droplets, which would impair the uniformity of the combustion. Branched-chain alkanes are preferred as they are much less prone to premature ignition, which causesknocking,than their straight-chain homologues. This propensity to premature ignition is measured by theoctane ratingof the fuel, where2,2,4-trimethylpentane(isooctane) has an arbitrary value of 100, andheptanehas a value of zero. Apart from their use as fuels, the middle alkanes are also goodsolventsfor nonpolar substances. Alkanes fromnonaneto, for instance,hexadecane(an alkane with sixteen carbon atoms) are liquids of higherviscosity,less and less suitable for use in gasoline. They form instead the major part ofdieselandaviation fuel.Diesel fuels are characterized by theircetane number,cetane being an old name for hexadecane. However, the higher melting points of these alkanes can cause problems at low temperatures and in polar regions, where the fuel becomes too thick to flow correctly.
Precursors to chemicals
[edit]By the process ofcracking,alkanes can be converted toalkenes.Simple alkenes are precursors to polymers, such aspolyethyleneandpolypropylene.When the cracking is taken to extremes, alkanes can be converted tocarbon black,which is a significant tire component.
Chlorination of methane gives chloromethanes, which are used as solvents and building blocks for complex compounds. Similarly treatment of methane with sulfur givescarbon disulfide.Still other chemicals are prepared by reaction withsulfur trioxideandnitric oxide
Other
[edit]Some light hydrocarbons are used asaerosol sprays.
Alkanes from hexadecane upwards form the most important components offuel oilandlubricating oil.In the latter function, they work at the same time as anti-corrosive agents, as their hydrophobic nature means that water cannot reach the metal surface. Many solid alkanes find use asparaffin wax,for example, incandles.This should not be confused however with truewax,which consists primarily ofesters.
Alkanes with a chain length of approximately 35 or more carbon atoms are found inbitumen,used, for example, in road surfacing. However, the higher alkanes have little value and are usually split into lower alkanes bycracking.
Hazards
[edit]Alkanes are highly flammable, but they have low toxicities. Methane "is toxicologically virtually inert." Alkanes can be asphyxiants and narcotic.[38]
See also
[edit]References
[edit]- ^abIUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "alkanes".doi:10.1351/goldbook.A00222
- ^Smith, Michael B.;March, Jerry(2007).Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.). New York: Wiley-Interscience. p. 23.ISBN978-0-471-72091-1.
- ^On-Line Encyclopedia of Integer Sequences(sequenceA000602in theOEIS) Number of n-node unrooted quartic trees; number of n-carbon alkanes C(n)H(2n+2) ignoring stereoisomers
- ^Alabugin, Igor V. (2016).Stereoelectronic effects: a bridge between structure and reactivity.Wiley.ISBN978-1-118-90637-8.OCLC957525299.
- ^IUPAC, Commission on Nomenclature of Organic Chemistry (1993)."R-2.2.1: Hydrocarbons".A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993).Blackwell Scientific.ISBN978-0-632-03488-8.Retrieved12 February2007.
- ^"Alkane Nomenclature".Archived fromthe originalon 2 February 2012.
- ^Thus, the ending "-diene" is applied in some cases where von Hofmann had "-ine"
- ^abReusch, William."Nomenclature – Alkanes".Virtual Textbook of Organic Chemistry.Archived fromthe originalon 21 May 2016.Retrieved5 April2007.
- ^Reusch, William."Examples of the IUPAC Rules in Practice".Virtual Textbook of Organic Chemistry.Archived fromthe originalon 21 May 2016.Retrieved5 April2007.
- ^"IUPAC Rules".www.chem.uiuc.edu.Retrieved13 August2018.
- ^"Definition of CYCLOALKANES".www.merriam-webster.com.Retrieved26 June2021.
- ^"Definition of NAPHTHENES".www.merriam-webster.com.Retrieved26 June2021.
- ^Mackay, Donald (14 March 2006).Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals.CRC Press. p. 206.ISBN1-4200-4439-7.
- ^"Pharmaceutical Chemistry"(PDF).Archived fromthe original(PDF)on 29 October 2013.Retrieved17 February2014.
- ^"13. Hydrocarbons | Textbooks".textbook.s-anand.net. Archived fromthe originalon 8 May 2011.Retrieved3 October2014.
- ^"Molecule Gallery - Alkanes".www.angelo.edu.Retrieved6 December2021.
- ^Allaby, Michael, ed. (1988). "Alkanes (paraffins)".Illustrated Dictionary of Science, Andromeda.Windmill Books (Andromeda International).
- ^abcdeR. T. Morrison; R. N. Boyd (1992).Organic Chemistry(6th ed.). Prentice Hall.ISBN978-0-13-643669-0.
- ^Boese, Roland; Weiss, Hans-Christoph; Bläser, Dieter (1 April 1999). "The Melting Point Alternation in the Short-Chainn-Alkanes: Single-Crystal X-Ray Analyses of Propane at 30 K and ofn-Butane ton-Nonane at 90 K ".Angewandte Chemie International Edition.38(7): 988–992.doi:10.1002/(SICI)1521-3773(19990401)38:7<988::AID-ANIE988>3.0.CO;2-0.ISSN1433-7851.
- ^"Solid methane".Visualization of Molecules and Crystal Structures.
- ^Ouellette, Robert J.; Rawn, J. David (1 January 2015)."Alkanes and Cycloalkanes".Principles of Organic Chemistry.Elsevier. pp. 65–94.doi:10.1016/b978-0-12-802444-7.00003-3.ISBN978-0-12-802444-7.
- ^Smith, Michael B.;March, Jerry(2007),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.), New York: Wiley-Interscience, p. 195,ISBN978-0-471-72091-1
- ^Silverstein, Robert M.; Webster, Francis X.; Kiemle, David J.; Bryce, David L. (2016).Spectrometric Identification of Organic Compounds(8th ed.). Wiley.ISBN978-0-470-61637-6.
- ^"Dodecane: IR Spectrum".NIST Chemistry WebBook.SRD 69.
- ^"Dodecane".NIST Chemistry WebBook.SRD 69.
- ^Olah, G.A.;Schlosberg, R.H. (1968). "Chemistry in Super Acids. I. Hydrogen Exchange and Polycondensation of Methane and Alkanes in FSO3H–SbF5( "Magic Acid" ) Solution. Protonation of Alkanes and the Intermediacy of CH5+and Related Hydrocarbon Ions. The High Chemical Reactivity of "Paraffins" in Ionic Solution Reactions ".Journal of the American Chemical Society.90(10): 2726–7.doi:10.1021/ja01012a066.
- ^Ji, Yurui; Mao, Guannan; Wang, Yingying; Bartlam, Mark (2013)."Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases".Frontiers in Microbiology.4:58.doi:10.3389/fmicb.2013.00058.PMC3604635.PMID23519435.
- ^Dashti, Narjes; Ali, Nedaa; Eliyas, Mohamed; Khanafer, Majida; Sorkhoh, Naser A.; Radwan, Samir S. (March 2015)."Most Hydrocarbonoclastic Bacteria in the Total Environment are Diazotrophic, which Highlights Their Value in the Bioremediation of Hydrocarbon Contaminants".Microbes and Environments.30(1): 70–75.doi:10.1264/jsme2.ME14090.ISSN1342-6311.PMC4356466.PMID25740314.
- ^Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine. "Fluorine Compounds, Organic".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a11_349.ISBN978-3527306732.
- ^Yu, Isaac F.; Wilson, Jake W.; Hartwig, John F. (2023). "Transition-Metal-Catalyzed Silylation and Borylation of C–H Bonds for the Synthesis and Functionalization of Complex Molecules".Chemical Reviews.123(19): 11619–63.doi:10.1021/acs.chemrev.3c00207.PMID37751601.S2CID263150991.
- ^abcAsinger, Friedrich (1967).Paraffins; Chemistry and Technology.Pergamon Press.OCLC556032.
- ^Lakdawalla, Emily."Titan: Arizona in an Icebox?".Archived fromthe originalon 6 April 2008.Retrieved21 January2004.
- ^Mumma, M.J.; Disanti, M.A.; dello Russo, N.; Fomenkova, M.; Magee-Sauer, K.; Kaminski, C.D.; D.X., Xie (1996). "Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin".Science.272(5266): 1310–4.Bibcode:1996Sci...272.1310M.doi:10.1126/science.272.5266.1310.PMID8650540.S2CID27362518.
- ^Janssen, P. H.; Kirs, M. (2008)."Structure of the Archaeal Community of the Rumen".Appl Environ Microbiol.74(12): 3619–25.Bibcode:2008ApEnM..74.3619J.doi:10.1128/AEM.02812-07.PMC2446570.PMID18424540.
- ^Buczkowski, Grzegorz; Bertelsmeier, Cleo (15 January 2017)."Invasive termites in a changing climate: A global perspective".Ecology and Evolution.7(3): 974–985.Bibcode:2017EcoEv...7..974B.doi:10.1002/ece3.2674.PMC5288252.PMID28168033.
- ^Blitz, Matt."Do Cow Farts Actually Contribute to Global Warming?".TodayIFoundOut.com.Retrieved11 April2018– via Gizmodo.
- ^"Natural Gas".Resources Library.National Geographic Society.Retrieved11 April2018.
- ^abSchmidt, Roland; Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut. "Hydrocarbons".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a13_227.pub3.ISBN978-3527306732.
- ^Smith, Michael B.;March, Jerry(2007),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.), New York: Wiley-Interscience, p. 1790,ISBN978-0-471-72091-1
- ^Noller, C.R. (1931). "n-Pentane".Organic Syntheses.11:84.doi:10.15227/orgsyn.011.0084.
- ^"Using propane as a fuel"(PDF).Archived fromthe original(PDF)on 12 October 2013.Retrieved27 November2012.
Further reading
[edit]- Virtual Textbook of Organic Chemistry
- A visualization of the crystal structures of alkanes up to nonan
- Redwood, Boverton (1911)..Encyclopædia Britannica.Vol. 20 (11th ed.). pp. 752–756.