Seawater
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water(< 0.05%) Brackish water(0.05–3%) Saline water(3–5%) Brine(> 5% up to 26%–28% max) |
| Bodies of water |
Seawater,orsea water,iswaterfrom aseaorocean.On average, seawater in the world's oceans has asalinityof about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) ofdissolved salts(predominantlysodium(Na+
) andchloride(Cl−
)ions). The average density at the surface is 1.025 kg/L. Seawater isdenserthan bothfresh waterand pure water (density 1.0 kg/L at 4 °C (39 °F)) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, itfreezesat about −2 °C (28 °F).[1]The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under anAntarcticglacier:the measured temperature was −2.6 °C (27.3 °F).[2]
SeawaterpHis typically limited to a range between 7.5 and 8.4.[3]However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.[4]
Properties
[edit]Salinity
[edit]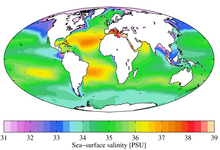
Although the vast majority of seawater has a salinity of between 31 and 38 g/kg, that is 3.1–3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with freshwater runoff from river mouths, near melting glaciers or vast amounts of precipitation (e.g.monsoon), seawater can be substantially less saline. The most saline open sea is theRed Sea,where high rates ofevaporation,lowprecipitationand low river run-off, and confined circulation result in unusually salty water. The salinity in isolated bodies of water can be considerably greater still – about ten times higher in the case of theDead Sea.Historically, several salinity scales were used to approximate the absolute salinity of seawater. A popular scale was the "Practical Salinity Scale" where salinity was measured in "practical salinity units (PSU)". The current standard for salinity is the "Reference Salinity" scale[6]with the salinity expressed in units of "g/kg".
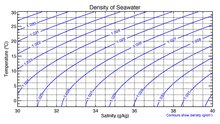
Density
[edit]Thedensityof surface seawater ranges from about 1020 to 1029 kg/m3,depending on the temperature and salinity. At a temperature of 25 °C, the salinity of 35 g/kg and 1 atm pressure, the density of seawater is 1023.6 kg/m3.[7][8]Deep in the ocean, under high pressure, seawater can reach a density of 1050 kg/m3or higher. The density of seawater also changes with salinity. Brines generated by seawater desalination plants can have salinities up to 120 g/kg. The density of typical seawater brine of 120 g/kg salinity at 25 °C and atmospheric pressure is 1088 kg/m3.[7][8]
pH value
[edit]ThepH valueat the surface of oceans in pre-industrial time (before 1850) was around 8.2.[9]Since then, it has been decreasing due to a human-caused process calledocean acidificationthat is related tocarbon dioxide emissions:Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05.[10]
The pH value of seawater is naturally as low as 7.8 in deep ocean waters as a result of degradation of organic matter in these waters.[11]It can be as high as 8.4 in surface waters in areas of highbiological productivity.[12]
Measurement of pH is complicated by thechemical propertiesof seawater, and several distinct pH scales exist inchemical oceanography.[13]There is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.[4]
Chemical composition
[edit]Seawater contains more dissolvedionsthan all types of freshwater.[14]However, the ratios of solutes differ dramatically. For instance, although seawater contains about 2.8 times morebicarbonatethan river water, thepercentageof bicarbonate in seawater as a ratio ofalldissolvedionsis far lower than in river water. Bicarbonate ions constitute 48% of river water solutes but only 0.14% for seawater.[14][15]Differences like these are due to the varyingresidence timesof seawater solutes;sodiumandchloridehave very long residence times, whilecalcium(vital forcarbonateformation) tends to precipitate much more quickly.[15]The most abundant dissolved ions in seawater are sodium, chloride,magnesium,sulfateand calcium.[16]Itsosmolarityis about 1000 mOsm/L.[17]
Small amounts of other substances are found, includingamino acidsat concentrations of up to 2 micrograms of nitrogen atoms per liter,[18]which are thought to have played a key role in theorigin of life.

55%,Na+
30.6%,SO2−
47.7%,Mg2+
3.7%,Ca2+
1.2%,K+
1.1%, Other 0.7%. Note that the diagram is only correct when in units of wt/wt, not wt/vol or vol/vol.
| Element | Percent by mass |
|---|---|
| Oxygen | 85.84 |
| Hydrogen | 10.82 |
| Chlorine | 1.94 |
| Sodium | 1.08 |
| Magnesium | 0.1292 |
| Sulfur | 0.091 |
| Calcium | 0.04 |
| Potassium | 0.04 |
| Bromine | 0.0067 |
| Carbon | 0.0028 |
| Component | Concentration (mol/kg) |
|---|---|
| H 2O |
53.6 |
| Cl− |
0.546 |
| Na+ |
0.469 |
| Mg2+ |
0.0528 |
| SO2− 4 |
0.0282 |
| Ca2+ |
0.0103 |
| K+ |
0.0102 |
| CT | 0.00206 |
| Br− |
0.000844 |
| BT | 0.000416 |
| Sr2+ |
0.000091 |
| F− |
0.000068 |
Microbial components
[edit]Research in 1957 by theScripps Institution of Oceanographysampled water in bothpelagicandneriticlocations in the Pacific Ocean. Direct microscopic counts and cultures were used, the direct counts in some cases showing up to 10 000 times that obtained from cultures. These differences were attributed to the occurrence of bacteria in aggregates, selective effects of the culture media, and the presence of inactive cells. A marked reduction in bacterial culture numbers was noted below thethermocline,but not by direct microscopic observation. Large numbers ofspirilli-like forms were seen by microscope but not under cultivation. The disparity in numbers obtained by the two methods is well known in this and other fields.[20]In the 1990s, improved techniques of detection and identification of microbes by probing just small snippets ofDNA,enabled researchers taking part in theCensus of Marine Lifeto identify thousands of previously unknown microbes usually present only in small numbers. This revealed a far greater diversity than previously suspected, so that a litre of seawater may hold more than 20,000 species.Mitchell Soginfrom theMarine Biological Laboratoryfeels that "the number of different kinds of bacteria in the oceans could eclipse five to 10 million."[21]
Bacteria are found at all depths in thewater column,as well as in the sediments, some being aerobic, others anaerobic. Most are free-swimming, but some exist assymbiontswithin other organisms – examples of these being bioluminescent bacteria.Cyanobacteriaplayed an important role in the evolution of ocean processes, enabling the development ofstromatolitesand oxygen in the atmosphere.
Some bacteria interact withdiatoms,and form a critical link in the cycling of silicon in the ocean. One anaerobic species,Thiomargarita namibiensis,plays an important part in the breakdown ofhydrogen sulfideeruptions from diatomaceous sediments off the Namibian coast, and generated by high rates ofphytoplanktongrowth in theBenguela Currentupwelling zone, eventually falling to the seafloor.
Bacteria-likeArchaeasurprised marine microbiologists by their survival and thriving in extreme environments, such as thehydrothermal ventson the ocean floor. Alkalotolerantmarine bacteriasuch asPseudomonasandVibriospp. survive in apHrange of 7.3 to 10.6, while some species will grow only at pH 10 to 10.6.[22]Archaea also exist in pelagic waters and may constitute as much as half the ocean'sbiomass,clearly playing an important part in oceanic processes.[23]In 2000 sediments from the ocean floor revealed a species of Archaea that breaks downmethane,an importantgreenhousegas and a major contributor to atmospheric warming.[24]Some bacteria break down the rocks of the sea floor, influencing seawater chemistry. Oil spills, and runoff containing human sewage and chemical pollutants have a marked effect on microbial life in the vicinity, as well as harbouring pathogens and toxins affecting all forms ofmarine life.The protistdinoflagellatesmay at certain times undergo population explosions called blooms orred tides,often after human-caused pollution. The process may producemetabolitesknown as biotoxins, which move along the ocean food chain, tainting higher-order animal consumers.
Pandoravirus salinus,a species of very large virus, with a genome much larger than that of any other virus species, was discovered in 2013. Like the other very large virusesMimivirusandMegavirus,Pandoravirusinfects amoebas, but its genome, containing 1.9 to 2.5 megabases of DNA, is twice as large as that ofMegavirus,and it differs greatly from the other large viruses in appearance and in genome structure.
In 2013 researchers fromAberdeen Universityannounced that they were starting a hunt for undiscovered chemicals in organisms that have evolved in deep sea trenches, hoping to find "the next generation" of antibiotics, anticipating an "antibiotic apocalypse" with a dearth of new infection-fighting drugs. The EU-funded research will start in theAtacama Trenchand then move on to search trenches off New Zealand and Antarctica.[25]
The ocean has a long history of human waste disposal on the assumption that its vast size makes it capable of absorbing and diluting all noxious material.[26] While this may be true on a small scale, the large amounts of sewage routinely dumped has damaged many coastal ecosystems, and rendered them life-threatening. Pathogenic viruses and bacteria occur in such waters, such asEscherichia coli,Vibrio choleraethe cause ofcholera,hepatitis A,hepatitis Eandpolio,along with protozoans causinggiardiasisandcryptosporidiosis.These pathogens are routinely present in the ballast water of large vessels, and are widely spread when the ballast is discharged.[27]
Other parameters
[edit]Thespeed of soundin seawater is about 1,500 m/s (whereas the speed of sound is usually around 330 m/s in air at roughly 101.3 kPa pressure, 1 atmosphere), and varies with water temperature, salinity, and pressure. Thethermal conductivityof seawater is 0.6 W/mK at 25 °C and a salinity of 35 g/kg.[28] The thermal conductivity decreases with increasing salinity and increases with increasing temperature.[29]
Origin and history
[edit]The water in the sea was thought to come from the Earth'svolcanoes,starting 4 billion years ago, released by degassing from molten rock.[30]: 24–25 More recent work suggests much of the Earth's water may come fromcomets.[31]
Scientific theoriesbehind the origins of sea salt started with SirEdmond Halleyin 1715, who proposed that salt and other minerals were carried into the sea by rivers after rainfall washed it out of the ground. Upon reaching the ocean, these salts concentrated as more salt arrived over time (seeHydrologic cycle). Halley noted that most lakes that do not have ocean outlets (such as theDead Seaand theCaspian Sea,seeendorheic basin), have high salt content. Halley termed this process "continental weathering".
Halley's theory was partly correct. In addition, sodium leached out of the ocean floor when the ocean formed. The presence of salt's other dominant ion, chloride, results fromoutgassingof chloride (ashydrochloric acid) with other gases from Earth's interior viavolcanosandhydrothermal vents.The sodium and chloride ions subsequently became the most abundant constituents of sea salt.
Ocean salinity has been stable for billions of years, most likely as a consequence of a chemical/tectonicsystem which removes as much salt as is deposited; for instance, sodium and chloride sinks includeevaporitedeposits, pore-water burial, and reactions with seafloorbasalts.[15]: 133
Human impacts
[edit]Climate change,rising levels ofcarbon dioxide in Earth's atmosphere,excess nutrients, and pollution in many forms are altering global oceanicgeochemistry.Rates of change for some aspects greatly exceed those in the historical and recent geological record. Major trends include an increasingacidity,reduced subsurface oxygen in both near-shore and pelagic waters, rising coastal nitrogen levels, and widespread increases inmercuryand persistent organic pollutants. Most of these perturbations are tied either directly or indirectly to human fossil fuel combustion, fertilizer, and industrial activity. Concentrations are projected to grow in coming decades, with negative impacts on ocean biota and other marine resources.[32]
One of the most striking features of this isocean acidification,resulting from increased CO2uptake of the oceans related to higher atmospheric concentration of CO2and higher temperatures,[33]because it severely affectscoral reefs,mollusks,echinodermsandcrustaceans(seecoral bleaching).
Seawater is a means of transportation throughout the world. Every day plenty of ships cross the ocean to deliver goods to various locations around the world. Seawater is a tool for countries to efficiently participate in international commercial trade and transportation, but each ship exhausts emissions that can harm marine life, air quality of coastal areas. Seawater transportation is one of the fastest growing human generated greenhouse gas emissions.[34]The emissions released from ships pose significant risks to human health in nearing areas as theoilandgasreleased from the operation of merchant ships decreases the air quality and causes morepollutionboth in the seawater and surrounding areas.[35]
Another human use of seawater that has been considered is the use of seawater foragriculturalpurposes. In areas with higher regions ofsand dunes,such asIsrael,the use of seawater forirrigationof plants would eliminate substantial costs associated with fresh water when it is not easily accessible.[36]Although it is not typical to usesalt wateras a means to grow plants as the salt gathers and ruins the surrounding soil, it has been proven to be successful in sand and gravel soils.[36]Large-scale desalination of seawater is another factor that would contribute to the success of agriculture farming in dry,desertenvironments.[36]One of the most successful plants in salt water agriculture is thehalophyte.The halophyte is a salt tolerant plant whose cells are resistant to the typically detrimental effects of salt in soil.[37]Theendodermisforces a higher level of salt filtration throughout the plant as it allows for the circulation of more water through the cells.[37]The cultivation of halophytes irrigated with salt water were used to growanimal feedforlivestock;however, the animals that were fed these plants consumed more water than those that did not.[37]Although agriculture from use of saltwater is still not recognized and used on a large scale, initial research has shown that there could be an opportunity to provide more crops in regions where agricultural farming is not usually feasible.
Human consumption
[edit]Accidentally consuming small quantities of clean seawater is not harmful, especially if the seawater is taken along with a larger quantity of fresh water. However, drinking seawater to maintain hydration is counterproductive; more water must be excreted to eliminate the salt (viaurine) than the amount of water obtained from the seawater itself.[38]In normal circumstances, it would be considered ill-advised to consume large amounts of unfiltered seawater.
Therenal systemactively regulates the levels of sodium and chloride in the blood within a very narrow range around 9 g/L (0.9% by mass).
In most open waters concentrations vary somewhat around typical values of about 3.5%, far higher than the body can tolerate and most beyond what the kidney can process. A point frequently overlooked in claims that the kidney can excrete NaCl inBalticconcentrations of 2% (in arguments to the contrary) is that the gut cannot absorb water at such concentrations, so that there is no benefit in drinking such water. The salinity of Baltic surface water, however, is never 2%. It is 0.9% or less, and thus never higher than that of bodily fluids. Drinking seawater temporarily increases blood's NaCl concentration. This signals thekidneyto excrete sodium, but seawater's sodium concentration is above the kidney's maximum concentrating ability. Eventually the blood's sodium concentration rises to toxic levels, removing water from cells and interfering withnerveconduction, ultimately producing fatalseizureandcardiac arrhythmia.[citation needed]
Survival manualsconsistently advise against drinking seawater.[39]A summary of 163life raftvoyages estimated the risk of death at 39% for those who drank seawater, compared to 3% for those who did not. The effect of seawater intake on rats confirmed the negative effects of drinking seawater when dehydrated.[40]
The temptation to drink seawater was greatest for sailors who had expended their supply of fresh water and were unable to capture enough rainwater for drinking. This frustration was described famously by a line fromSamuel Taylor Coleridge'sThe Rime of the Ancient Mariner:
Water, water, everywhere,
And all the boards did shrink;
Water, water, everywhere,
Nor any drop to drink.
Although humans cannot survive on seawater, some people claim that up to two cups a day, mixed with fresh water in a 2:3 ratio, produces no ill effect. The French physicianAlain Bombardsurvived an ocean crossing in a small Zodiak rubber boat using mainly raw fish meat, which contains about 40% water (like most living tissues), as well as small amounts of seawater and other provisions harvested from the ocean. His findings were challenged, but an alternative explanation was not given. In his 1948 bookThe Kon-Tiki Expedition,Thor Heyerdahlreported drinking seawater mixed with fresh in a 2:3 ratio during the 1947 expedition.[41]A few years later, another adventurer,William Willis,claimed to have drunk two cups of seawater and one cup of fresh per day for 70 days without ill effect when he lost part of his water supply.[42]
During the 18th century,Richard Russelladvocated the medical use of this practice in the UK,[43]andRené Quintonexpanded the advocation of this practice to other countries, notably France, in the 20th century. Currently, it is widely practiced in Nicaragua and other countries, supposedly taking advantage of the latest medical discoveries.[44][45]
Most oceangoing vesselsdesalinatepotablewater from seawater using processes such asvacuum distillationormulti-stage flash distillationin anevaporator,or, more recently,reverse osmosis.These energy-intensive processes were not usually available during theAge of Sail.Larger sailing warships with large crews, such asNelson'sHMSVictory,were fitted with distilling apparatus in theirgalleys.[46] Animals such as fish, whales,sea turtles,andseabirds,such as penguins andalbatrosses,have adapted to living in a high-saline habitat. For example, sea turtles and saltwater crocodiles remove excess salt from their bodies through theirtear ducts.[47]
Mineral extraction
[edit]Minerals have been extracted from seawater since ancient times. Currently the four most concentrated metals –Na,Mg,CaandK– are commercially extracted from seawater.[48]During 2015 in theUS63% ofmagnesiumproduction came from seawater and brines.[49]Bromineis also produced from seawater inChinaand Japan.[50]Lithiumextraction from seawater was tried in the 1970s, but the tests were soon abandoned. The idea of extractinguraniumfrom seawater has been considered at least from the 1960s, but only a few grams of uranium were extracted inJapanin the late 1990s.[51]The main issue is not one of technological feasibility but that current prices on theuranium marketfor uranium from other sources are about three to five times lower than the lowest price achieved by seawater extraction.[52][53]Similar issues hamper the use ofreprocessed uraniumand are often brought forth againstnuclear reprocessingand the manufacturing ofMOX fuelas economically unviable.
The future of mineral and element extractions
[edit]In order for seawater mineral and element extractions to take place while taking close consideration of sustainable practices, it is necessary for monitored management systems to be put in place. This requires management of ocean areas and their conditions,environmental planning,structured guidelines to ensure that extractions are controlled, regular assessments of the condition of the sea post-extraction, and constant monitoring.[54]The use of technology, such asunderwater drones,can facilitate sustainable extractions.[55]The use of low-carbon infrastructure would also allow for more sustainable extraction processes while reducing the carbon footprint from mineral extractions.[55]

Another practice that is being considered closely is the process ofdesalinationin order to achieve a more sustainable water supply from seawater. Although desalination also comes with environmental concerns, such as costs and resources, researchers are working closely to determine more sustainable practices, such as creating more productive water plants that can deal with larger water supplies in areas where these plans weren't always available.[56]Although seawater extractions can benefit society greatly, it is crucial to consider the environmental impact and to ensure that all extractions are conducted in a way that acknowledges and considers the associated risks to the sustainability of seawater ecosystems.
Standard
[edit]ASTM Internationalhas an international standard forartificial seawater:ASTM D1141-98 (Original Standard ASTM D1141-52). It is used in many research testing labs as a reproducible solution for seawater such as tests on corrosion, oil contamination, and detergency evaluation.[57]
Ecosystems
[edit]The minerals found in seawater can also play an important role in the ocean and its ecosystem's food cycle. For example, theSouthern Oceancontributes greatly to the environmentalcarbon cycle.Given that this body of water does not contain high levels ofiron,the deficiency impacts the marine life living in its waters. As a result, this ocean is not able to produce as muchphytoplanktonwhich hinders the first source of the marine food chain.[58]One of the main types of phytoplankton arediatomswhich is the primary food source ofAntarctic krill.As the cycle continues, various larger sea animals feed off of Antarctic krill, but since there is a shortage of iron from the initial phytoplankton/diatoms, then these larger species also lack iron. The larger sea animals includeBaleen Whalessuch as theBlue WhaleandFin Whale.[58]These whales not only rely on iron for a balance of minerals within their diet, but it also impacts the amount of iron that is regenerated back into the ocean. The whale's excretions also contain the absorbed iron which would allow iron to be reinserted into the ocean’s ecosystem. Overall, one mineral deficiency such as iron in the Southern Ocean can spark a significant chain of disturbances within the marine ecosystems which demonstrates the important role that seawater plays in thefood chain.
Upon further analysis of the dynamic relationship between diatoms, krill, and baleen whales, fecal samples of baleen whales were examined in Antarctic seawater.[58]The findings included that iron concentrations were 10 million times higher than those found in Antarctic seawater, and krill was found consistently throughout their feces which is an indicator that krill is in whale diets.[58]Antarctic krill had an average iron level of 174.3mg/kg dry weight, but the iron in the krill varied from 12 to 174 mg/kg dry weight.[58]The average iron concentration of the muscular tissue of blue whales and fin whales was 173 mg/kg dry weight, which demonstrates that the large marine mammals are important to marine ecosystems such as they are to the Southern Ocean.[58]In fact, to have more whales in the ocean could heighten the amount of iron in seawater through their excretions which would promote a better ecosystem.
Krill and baleen whales act as large iron reservoirs in seawater in the Southern Ocean. Krill can retain up to 24% of iron found on surface waters within its range.[58]The process of krill feeding on diatoms releases iron into seawater, highlighting them as an important part of the ocean'siron cycle.The advantageous relationship between krill and baleen whales increases the amount of iron that can be recycled and stored in seawater.[58]Apositive feedback loopis created, increasing the overall productivity of marine life in the Southern Ocean.
Organisms of all sizes play a significant role in the balance of marine ecosystems with both the largest and smallest inhabitants contributing equally to recycling nutrients in seawater. Prioritizing the recovery of whale populations because they boost the overall productivity in marine ecosystems as well as increasing iron levels in seawater would allow for a balanced and productive system for the ocean. However, a more in depth study is required to understand the benefits of whale feces as a fertilizer and to provide further insight in iron recycling in the Southern Ocean.[58]Projects on the management of ecosystems and conservation are vital for advancing knowledge of marine ecology.
Environmental impact and sustainability
[edit]Like any mineral extraction practices, there are environmental advantages and disadvantages.CobaltandLithiumare two key metals that can be used for aiding with more environmentally friendly technologies above ground, such as powering batteries that energizeelectric vehiclesor creatingwind power.[59]An environmentally friendly approach to mining that allows for more sustainability would be to extract these metals from the seafloor.Lithium miningfrom the seafloor at mass quantities could provide a substantial amount of renewable metals to promote more environmentally friendly practices in society to reduce humans'carbon footprint.Lithium mining from the seafloor could be successful, but its success would be dependent on more productiverecyclingpractices above ground.[60]

There are also risks that come with extracting from the seafloor. Many biodiverse species have long lifespans on the seafloor, which means that their reproduction takes more time.[54]Similarly to fish harvesting from the seafloor, the extraction of minerals in large amounts, too quickly, without proper protocols, can result in a disruption of the underwater ecosystems.[54]Contrarily, this would have the opposite effect and prevent mineral extractions from being a long-term sustainable practice, and would result in a shortage of required metals. Any seawater mineral extractions also risk disrupting the habitat of the underwater life that is dependent on the uninterrupted ecosystem within their environment as disturbances can have significant disturbances on animal communities.[54]
See also
[edit]- Brine– Concentrated solution of salt in water
- Brine mining– Extracting materials from saltwater
- Brackish water– Water with salinity between freshwater and seawater
- Fresh water– Naturally occurring water with low amounts of dissolved salts
- Ocean color– Explanation of the color of oceans and ocean color remote sensing
- Saline water– Water that contains a high concentration of dissolved salts
- Sea ice– Outcome of seawater as it freezes
- Seawater pH– Measure of the level of acidity or basicity of an aqueous solution
- Surface tension of seawater– Tendency of a liquid surface to shrink to reduce surface area
- Thalassotherapy
- Thermohaline circulation– Part of large-scale ocean circulation
- CORA dataset– Oceanographic temperature and salinity dataset global ocean salinity
References
[edit]- ^"U.S. Office of Naval Research Ocean, Water: Temperature".Archived fromthe originalon 12 December 2007.
- ^Sylte, Gudrun Urd (24 May 2010)."Den aller kaldaste havstraumen".forskning.no(in Norwegian). Archived fromthe originalon 6 March 2012.Retrieved24 May2010.
- ^Chester, Jickells, Roy, Tim (2012).Marine Geochemistry.Blackwell Publishing.ISBN978-1-118-34907-6.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^abStumm, W, Morgan, J. J. (1981)Aquatic Chemistry, An Introduction Emphasizing Chemical Equilibria in Natural Waters.John Wiley & Sons. pp. 414–416.ISBN0471048313.
- ^"World Ocean Atlas 2009".NOAA.Retrieved5 December2012.
- ^Millero, Frank J.; Feistel, Rainer; Wright, Daniel G.; McDougall, Trevor J. (January 2008). "The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale".Deep Sea Research Part I: Oceanographic Research Papers.55(1): 50–72.Bibcode:2008DSRI...55...50M.doi:10.1016/j.dsr.2007.10.001.
- ^abNayar, Kishor G.; Sharqawy, Mostafa H.; Banchik, Leonardo D.; Lienhard V, John H. (July 2016)."Thermophysical properties of seawater: A review and new correlations that include pressure dependence".Desalination.390:1–24.Bibcode:2016Desal.390....1N.doi:10.1016/j.desal.2016.02.024.hdl:1721.1/106794.
- ^ab"Thermophysical properties of seawater".Department of Mechanical Engineering,Massachusetts Institute of Technology.Retrieved24 February2017.
- ^Arias, P.A., N. Bellouin, E. Coppola, R.G. Jones, G. Krinner, J. Marotzke, V. Naik, M.D. Palmer, G.-K. Plattner, J. Rogelj, M. Rojas, J. Sillmann, T. Storelvmo, P.W. Thorne, B. Trewin, K. Achuta Rao, B. Adhikary, R.P. Allan, K. Armour, G. Bala, R. Barimalala, S. Berger, J.G. Canadell, C. Cassou, A. Cherchi, W. Collins, W.D. Collins, S.L. Connors, S. Corti, F. Cruz, F.J. Dentener, C. Dereczynski, A. Di Luca, A. Diongue Niang, F.J. Doblas-Reyes, A. Dosio, H. Douville, F. Engelbrecht, V. Eyring, E. Fischer, P. Forster, B. Fox-Kemper, J.S. Fuglestvedt, J.C. Fyfe, et al., 2021:Technical SummaryArchived21 July 2022 at theWayback Machine.InClimate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate ChangeArchived9 August 2021 at theWayback Machine[Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 33−144.
- ^Terhaar, Jens; Frölicher, Thomas L.; Joos, Fortunat (2023)."Ocean acidification in emission-driven temperature stabilization scenarios: the role of TCRE and non-CO2greenhouse gases ".Environmental Research Letters.18(2): 024033.Bibcode:2023ERL....18b4033T.doi:10.1088/1748-9326/acaf91.ISSN1748-9326.S2CID255431338.
- ^Emerson, Steven; Hedges, John (24 April 2008). "Chapter 4: Carbonate chemistry".Chemical Oceanography and the Marine Carbon Cycle(1 ed.). Cambridge University Press.doi:10.1017/cbo9780511793202.ISBN978-0-521-83313-4.
- ^Chester, R.; Jickells, Tim (2012). "Chapter 9: Nutrients, oxygen, organic carbon and the carbon cycle in seawater".Marine geochemistry(3rd ed.). Chichester, West Sussex, UK: Wiley/Blackwell.ISBN978-1-118-34909-0.OCLC781078031.
- ^Zeebe, R. E. and Wolf-Gladrow, D. (2001)CO2in seawater: equilibrium, kinetics, isotopes,Elsevier Science B.V., Amsterdam, NetherlandsISBN0-444-50946-1
- ^abGale, Thomson."Ocean Chemical Processes".Retrieved2 December2006.
- ^abcPinet, Paul R. (1996).Invitation to Oceanography.St. Paul: West Publishing Company. pp. 126, 134–135.ISBN978-0-314-06339-7.
- ^Hogan, C. Michael (2010)."Calcium",eds. A. Jorgensen, C. Cleveland.Encyclopedia of Earth.Some evidence shows the potential for fairly regular ratios of elements maintained across surface oceans in a phenomenon known as theRedfield Ratio.National Council for Science and the Environment.
- ^"Osmolarity of sea water - Biosphere - BNID 100802".bionumbers.hms.harvard.edu.
- ^Tada, K.; Tada, M.; Maita, Y. (1998)."Dissolved free amino acids in coastal seawater using a modified fluorometric method"(PDF).Journal of Oceanography.54(4): 313–321.Bibcode:1998JOce...54..313T.doi:10.1007/BF02742615.S2CID26231863.Archived fromthe original(PDF)on 21 January 2021.Retrieved28 August2015.
- ^DOE (1994)."5"(PDF).In A. G. Dickson; C. Goyet (eds.).Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water.2. ORNL/CDIAC-74. Archived fromthe original(PDF)on 25 May 2011.Retrieved18 May2006.
- ^Jannasch, Holger W.; Jones, Galen E. (1959)."Bacterial Populations in Sea Water as Determined by Different Methods of Enumeration".Limnology and Oceanography.4(2): 128–139.Bibcode:1959LimOc...4..128J.doi:10.4319/lo.1959.4.2.0128.
- ^"Ocean Microbe Census Discovers Diverse World of Rare Bacteria".ScienceDaily.2 September 2006.Retrieved13 May2013.
- ^Maeda, M.; Taga, N. (31 March 1980)."Alkalotolerant and Alkalophilic Bacteria in Seawater".Marine Ecology Progress Series.2:105–108.Bibcode:1980MEPS....2..105M.doi:10.3354/meps002105.
- ^Cheung, Louisa (31 July 2006)."Thousands of microbes in one gulp".BBC News.Retrieved13 May2013.
- ^Leslie, Mitchell (5 October 2000)."The Case of the Missing Methane".ScienceNOW.American Association for the Advancement of Science. Archived fromthe originalon 26 May 2013.Retrieved13 May2013.
- ^"Antibiotics search to focus on sea bed".BBC News.14 February 2013.Retrieved13 May2013.
- ^Panel On Radioactivity In The Marine Environment, National Research Council (U.S.) (1971).Radioactivity in the marine environment - National Academies, 1971.National Academies. p.36.ISBN9780309018654.
- ^Hoyle, Brian D.; Robinson, Richard."Microbes in the Ocean".Water Encyclopedia.
- ^Sharqawy, Mostafa H.; Lienhard V, John H.; Zubair, Syed M. (April 2010)."The thermophysical properties of seawater: A review of existing correlations and data"(PDF).Desalination and Water Treatment.16(1–3): 354–380.Bibcode:2010DWatT..16..354S.doi:10.5004/dwt.2010.1079.hdl:1721.1/69157.S2CID93362418.
- ^"Thermal conductivity of seawater and its concentrates".Retrieved17 October2010.
- ^Stow, Dorrik (2004).Encyclopedia of the Oceans.Oxford University Press.ISBN978-0-19-860687-1.
- ^Cowen, Ron (5 October 2011)."Comets take pole position as water bearers".Nature.doi:10.1038/news.2011.579.Retrieved10 September2013.
- ^Doney, Scott C.(18 June 2010). "The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry".Science.328(5985): 1512–1516.Bibcode:2010Sci...328.1512D.doi:10.1126/science.1185198.PMID20558706.S2CID8792396.
- ^Doney, Scott C.; Fabry, Victoria J.; Feely, Richard A.;Kleypas, Joan A.(1 January 2009). "Ocean Acidification: The Other CO2 Problem".Annual Review of Marine Science.1(1): 169–192.Bibcode:2009ARMS....1..169D.doi:10.1146/annurev.marine.010908.163834.PMID21141034.S2CID402398.
- ^Vaishnav, Parth (2014)."Greenhouse Gas Emissions from International Transport".Issues in Science and Technology.30(2): 25–28.ISSN0748-5492.JSTOR43315842.
- ^Iodice, Paolo; Langella, Giuseppe; Amoresano, Amedeo (2017)."A numerical approach to assess air pollution by ship engines in manoeuvring mode and fuel switch conditions".Energy & Environment.28(8): 827–845.Bibcode:2017EnEnv..28..827I.doi:10.1177/0958305X17734050.ISSN0958-305X.JSTOR90015687.
- ^abcBoyko, Hugo (1967)."Salt-Water Agriculture".Scientific American.216(3): 89–101.Bibcode:1967SciAm.216c..89B.doi:10.1038/scientificamerican0367-89.ISSN0036-8733.JSTOR24931436.
- ^abcGlenn, Edward P.; Brown, J. Jed; O’Leary, James W. (1998)."Irrigating Crops with Seawater".Scientific American.279(2): 76–81.Bibcode:1998SciAm.279b..76G.doi:10.1038/scientificamerican0898-76.ISSN0036-8733.JSTOR26070601.
- ^"Can humans drink seawater?".National Ocean Service(NOAA). 26 February 2021.
- ^"29"(PDF).Shipboard Medicine.Archived fromthe original(PDF)on 22 June 2007.Retrieved17 October2010.
- ^Etzion, Z.; Yagil, R. (1987). "Metabolic effects in rats drinking increasing concentrations of seawater".Comp Biochem Physiol A.86(1): 49–55.doi:10.1016/0300-9629(87)90275-1.PMID2881655.
- ^Heyerdahl, Thor; Lyon, F. H. (translator) (1950).Kon-Tiki: Across the Pacific by Raft.Rand McNally & Company, Chicago, Ill.
- ^King, Dean (2004).Skeletons on the Zahara: a true story of survival.New York: Back Bay Books. p. 74.ISBN978-0-316-15935-7.
- ^"History of the 18th century medical use of sea water in Britain".drinkingseawater.com.
- ^Martin, Francisco (2020). "chapter 12: Medical use of sea water in Nicaragua".Drinking Sea Water.ISBN979-8666741658.
- ^"Medical use of sea water in Nicaragua".drinkingseawater.com.
- ^Rippon, P. M., Commander, RN (1998).The evolution of engineering in the Royal Navy.Vol. 1: 1827–1939. Spellmount. pp. 78–79.ISBN978-0-946771-55-4.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Dennis, Jerry (23 September 2014).The Bird in the Waterfall: Exploring the Wonders of Water.Diversion Books.ISBN9781940941547.
- ^Loganathan, Paripurnanda; Naidu, Gayathri; Vigneswaran, Saravanamuthu (2017)."Mining valuable minerals from seawater: a critical review".Environmental Science: Water Research & Technology.3(1): 37–53.doi:10.1039/C6EW00268D.hdl:10453/121701.
- ^Campbell, Keith."Over 40 minerals and metals contained in seawater, their extraction likely to increase in the future".Mining Weekly.Retrieved8 February2023.
- ^"Global Bromine Industry And Its Outlook"(PDF).
- ^Ugo Bardi (2008)."Mining the Oceans: Can We Extract Minerals from Seawater?".theoildrum.com.Retrieved8 February2023.
- ^"Viability of Uranium Extraction from Sea Water".
- ^"Cost-effective method of extracting uranium from seawater promises limitless nuclear power".14 June 2018.
- ^abcdLevin, Lisa A. (2019)."SUSTAINABILITY IN DEEP WATER: The Challenges of Climate Change, Human Pressures, and Biodiversity Conservation".Oceanography.32(2): 170–180.doi:10.5670/oceanog.2019.224.ISSN1042-8275.JSTOR26651193.
- ^abSantos, Eleonora (16 April 2024)."Innovative solutions for coastal and offshore infrastructure in seawater mining: Enhancing efficiency and environmental performance".Desalination.575:117282.Bibcode:2024Desal.57517282S.doi:10.1016/j.desal.2023.117282.ISSN0011-9164.
- ^Ayaz, Muhammad; Namazi, M. A.; Din, M. Ammad ud; Ershath, M. I. Mohamed; Mansour, Ali; Aggoune, el-Hadi M. (15 October 2022)."Sustainable seawater desalination: Current status, environmental implications and future expectations".Desalination.540:116022.Bibcode:2022Desal.54016022A.doi:10.1016/j.desal.2022.116022.ISSN0011-9164.
- ^"ASTM D1141-98(2013)".ASTM.Retrieved17 August2013.
- ^abcdefghiNicol, Stephen; Bowie, Andrew; Jarman, Simon; Lannuzel, Delphine; Meiners, Klaus M; Van Der Merwe, Pier (13 May 2010)."Southern Ocean iron fertilization by baleen whales and Antarctic krill".Fish and Fisheries.11(2): 203–209.Bibcode:2010AqFF...11..203N.doi:10.1111/j.1467-2979.2010.00356.x.ISSN1467-2960.
- ^McCarthy, Rebecca (2020)."Deep Sea Rush: With valuable metals on the ocean floor, speculators are circling".The Baffler(54): 114–124.ISSN1059-9789.JSTOR26975674.
- ^Bardi, Ugo (April 2010)."Extracting Minerals from Seawater: An Energy Analysis".Sustainability.2(4): 980–992.doi:10.3390/su2040980.hdl:2158/779042.ISSN2071-1050.
External links
[edit]Tables
- Tables and software for thermophysical properties of seawater,MIT
- G. W. C Kaye, T. H. Laby (1995). "Physical properties of sea water".Tables of physical and chemical constants(16th ed.). Archived fromthe originalon 8 May 2019.