Sulfur
 | ||||||||||||||||||||||||||||||||||||
| Sulfur | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative name | Sulphur (British spelling) | |||||||||||||||||||||||||||||||||||
| Allotropes | seeAllotropes of sulfur | |||||||||||||||||||||||||||||||||||
| Appearance | Lemon yellowsinteredmicrocrystals | |||||||||||||||||||||||||||||||||||
| Standard atomic weightAr°(S) | ||||||||||||||||||||||||||||||||||||
| Sulfur in theperiodic table | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Atomic number(Z) | 16 | |||||||||||||||||||||||||||||||||||
| Group | group 16 (chalcogens) | |||||||||||||||||||||||||||||||||||
| Period | period 3 | |||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s23p4 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 6 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| PhaseatSTP | solid | |||||||||||||||||||||||||||||||||||
| Melting point | alpha (α-S8): 388.36K(115.21 °C, 239.38 °F) | |||||||||||||||||||||||||||||||||||
| Boiling point | 717.8 K (444.6 °C, 832.3 °F) | |||||||||||||||||||||||||||||||||||
| Density(nearr.t.) | alpha (α-S8): 2.07 g/cm3 beta (β-S8): 1.96 g/cm3 gamma (γ-S8): 1.92 g/cm3 | |||||||||||||||||||||||||||||||||||
| when liquid (atm.p.) | 1.819 g/cm3 | |||||||||||||||||||||||||||||||||||
| Critical point | 1314 K, 20.7 MPa | |||||||||||||||||||||||||||||||||||
| Heat of fusion | beta (β-S8): 1.727kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | beta (β-S8): 45 kJ/mol | |||||||||||||||||||||||||||||||||||
| Molar heat capacity | 22.75 J/(mol·K) | |||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | −2,−1,0,+1,+2,+3,+4,+5,+6(a stronglyacidicoxide) | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.58 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||
| Covalent radius | 105±3pm | |||||||||||||||||||||||||||||||||||
| Van der Waals radius | 180 pm | |||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||
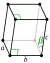
| Crystal structure | alpha (α-S8): orthorhombic(oF128) | |||||||||||||||||||||||||||||||||||
| Lattice constants | a= 1.0460 nm b= 1.2861 nm c= 2.4481 nm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||
| Crystal structure | beta (β-S8): monoclinic(mP48) | |||||||||||||||||||||||||||||||||||
| Lattice constants | a= 1.0923 nm b= 1.0851 nm c= 1.0787 nm β = 95.905° (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.205 W/(m⋅K) (amorphous) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 2×1015Ω⋅m (at 20 °C) (amorphous) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[4] | |||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | alpha (α-S8):−15.5×10−6cm3/mol (298 K)[5] | |||||||||||||||||||||||||||||||||||
| Bulk modulus | 7.7 GPa | |||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | |||||||||||||||||||||||||||||||||||
| CAS Number | 7704-34-9 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Discovery | before 2000 BCE[6] | |||||||||||||||||||||||||||||||||||
| Recognized as anelementby | Antoine Lavoisier(1777) | |||||||||||||||||||||||||||||||||||
| Isotopes of sulfur | ||||||||||||||||||||||||||||||||||||
34S abundances vary greatly (between 3.96 and 4.77 percent) in natural samples. | ||||||||||||||||||||||||||||||||||||
Sulfur(also spelledsulphurinBritish English) is achemical element;it hassymbolSandatomic number16. It isabundant,multivalentandnonmetallic.Undernormal conditions,sulfur atoms formcyclic octatomic moleculeswith the chemical formulaS8.Elemental sulfur is a bright yellow,crystallinesolid atroom temperature.
Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure,nativeform, sulfur on Earth usually occurs assulfideandsulfate minerals.Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses inancient India,ancient Greece,China,andancient Egypt.Historically and in literature sulfur is also calledbrimstone,[7]which means "burning stone".[8]Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants fromnatural gasandpetroleum.[9][10]The greatest commercial use of the element is the production ofsulfuric acidfor sulfate and phosphatefertilizers,and other chemical processes. Sulfur is used inmatches,insecticides,andfungicides.Many sulfur compounds are odoriferous, and the smells of odorized natural gas,skunkscent,bad breath,grapefruit,andgarlicare due toorganosulfurcompounds.Hydrogen sulfidegives the characteristic odor to rotting eggs and other biological processes.
Sulfur is anessential elementfor all life, almost always in the form oforganosulfur compoundsor metal sulfides.Amino acids(twoproteinogenic:cysteineandmethionine,and many othernon-coded:cystine,taurine,etc.) and two vitamins (biotinandthiamine) are organosulfur compounds crucial for life. Manycofactorsalso contain sulfur, includingglutathione,andiron–sulfur proteins.Disulfides,S–S bonds, confer mechanical strength and insolubility of the (among others) proteinkeratin,found in outer skin, hair, and feathers. Sulfur is one of the core chemical elements needed forbiochemicalfunctioning and is an elementalmacronutrientfor all living organisms.
Characteristics
[edit]Physical properties
[edit]
Sulfur forms several polyatomic molecules. The best-known allotrope isoctasulfur,cyclo-S8.Thepoint groupof cyclo-S8is D4dand its dipole moment is 0 D.[11]Octasulfur is a soft, bright-yellow solid that is odorless.[a]It melts at 115.21 °C (239.38 °F),[b]boils at 444.6 °C (832.3 °F).[7]At 95.2 °C (203.4 °F), below its melting temperature, cyclo-octasulfur begins slow changing from α-octasulfur to the β-polymorph.[13]The structure of the S8ring is virtually unchanged by this phase change, which affects the intermolecular interactions. Cooling of molten sulfur gives freezing point in 119.6 °C (247.3 °F),[14]as it predominantly consists of the β-S8molecules.[c]Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increasedviscositydue to the formation ofpolymers.[13]At higher temperatures, the viscosity decreases as depolymerization occurs. Molten sulfur assumes a dark red color above 200 °C (392 °F). The density of sulfur is about 2 g/cm3,depending on the allotrope; all of the stable allotropes are excellent electrical insulators.
Sulfursublimesmore or less between 20 °C (68 °F) and 50 °C (122 °F).[18]
Sulfur is insoluble in water but soluble incarbon disulfideand, to a lesser extent, in othernonpolarorganic solvents, such asbenzeneandtoluene.
Chemical properties
[edit]Under normal conditions, sulfurhydrolyzesvery slowly to mainly formhydrogen sulfideandsulfuric acid:
8+ 4H
2O→ 3H
2S+H
2SO
4
The reaction involves adsorption of protons ontoS
8clusters, followed bydisproportionationinto the reaction products.[19]
The second, fourth and sixthionization energiesof sulfur are 2252 kJ/mol, 4556 kJ/mol and 8495.8 kJ/mol, respectively. A composition of products of sulfur's reactions with oxidants (and its oxidation state) depends on that whether releasing out of a reaction energy overcomes these thresholds. Applyingcatalystsand / orsupply of outer energymay vary sulfur's oxidation state and a composition of reaction products. While reaction between sulfur and oxygen at normal conditions gives sulfur dioxide (oxidation state +4), formation ofsulfur trioxide(oxidation state +6) requires temperature 400–600 °C (750–1,100 °F) and presence of a catalyst.
In reactions with elements of lesserelectronegativity,it reacts as an oxidant and forms sulfides, where it has oxidation state −2.
Sulfur reacts with nearly all other elements with the exception of the noble gases, even with the notoriously unreactive metaliridium(yieldingiridium disulfide).[20]Some of those reactions need elevated temperatures.[21]
Allotropes
[edit]
Sulfur forms over 30 solidallotropes,more than any other element.[22]Besides S8,several other rings are known.[23]Removing one atom from the crown gives S7,which is of a deeper yellow than S8.HPLCanalysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8,but with S7and small amounts of S6.[24]Larger rings have been prepared, including S12and S18.[25][26]
Amorphousor "plastic" sulfur is produced by rapid cooling of molten sulfur—for example, by pouring it into cold water.X-ray crystallographystudies show that the amorphous form may have ahelicalstructure with eight atoms per turn. The long coiled polymeric molecules make the brownish substanceelastic,and in bulk this form has the feel of crude rubber. This form ismetastableat room temperature and gradually reverts to the crystalline molecular allotrope, which is no longer elastic. This process happens within a matter of hours to days, but can be rapidly catalyzed.
Isotopes
[edit]Sulfur has 23 knownisotopes,four of which are stable:32S (94.99%±0.26%),33S (0.75%±0.02%),34S (4.25%±0.24%), and36S (0.01%±0.01%).[27][28]Other than35S, with ahalf-lifeof 87 days, theradioactiveisotopes of sulfur have half-lives less than 3 hours.
The preponderance of32S is explained by its production in the so-called alpha-process (one of the main classes of nuclear fusion reactions) in exploding stars. Other stable sulfur isotopes are produced in the bypass processes related with34Ar, and their composition depends on a type of a stellar explosion. For example, proportionally more33S comes fromnovaethan fromsupernovae.[29]
On the planet Earth the sulfur isotopic composition was determined by the Sun. Though it is assumed that the distribution of different sulfur isotopes should be more or less equal, it has been found that proportions of two most abundant sulfur isotopes32S and34S varies in different samples. Assaying of these isotopes ratio (δ34S) in the samples allows to make suggestions about their chemical history, and with support of other methods, it allows to age-date the samples, estimate temperature of equilibrium between ore and water, determine pH and oxygen fugacity, identify the activity of sulfate-reducing bacteria in the time of formation of the sample, or suggest the main sources of sulfur in ecosystems.[30]However, there are ongoing discussions about what is the real reason of the δ34S shifts, biological activity or postdeposital alteration.[31]
For example, whensulfide mineralsare precipitated, isotopic equilibration among solids and liquid may cause small differences in theδ34Svalues of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. Theδ13Cand δ34S of coexistingcarbonate mineralsand sulfides can be used to determine thepHand oxygenfugacityof the ore-bearing fluid during ore formation.
Scientists measure thesulfur isotopesofmineralsin rocks andsedimentsto study theredoxconditions in the oceans in the past.Sulfate-reducing bacteriain marine sediment fractionatesulfur isotopesas they take insulfateand producesulfide.Prior to the 2010s, it was thought that sulfate reduction could fractionatesulfur isotopesup to 46permil[32]and fractionation larger than 46 permil recorded in sediments must be due todisproportionationof sulfur compounds in the sediment. This view has changed since the 2010s as experiments show thatsulfate-reducing bacteriacan fractionate to 66 permil.[33]As substrates for disproportionation are limited by the product ofsulfate reduction,the isotopic effect of disproportionation should be less than 16 permil in most sedimentary settings.[34]
In mostforestecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites contribute some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer inhydrologicstudies. Differences in thenatural abundancescan be used in systems where there is sufficient variation in the34S of ecosystem components.Rocky Mountainlakes thought to be dominated by atmospheric sources of sulfate have been found to have measurably different34S values than lakes believed to be dominated by watershed sources of sulfate.
The radioactive35S is formed incosmic ray spallationof the atmospheric40Ar.This fact may be used for proving the presence of recent (not more than 1 year) atmospheric sediments in various things. This isotope may be obtained artificially by different ways. In practice, the reaction35Cl+n→35S +pis used by irradiatingpotassium chloridewith neutrons.[35]The isotope35S is used in various sulfur-containing compounds as aradioactive tracerfor many biological studies, for example, theHershey-Chase experiment.
Because of the weakbeta activityof35S, its compounds are relatively safe as long as they are not ingested or absorbed by the body.[36]
Natural occurrence
[edit]



32S is created inside massive stars, at a depth where the temperature exceeds 2.5×109K, by thefusionof one nucleus of silicon plus one nucleus of helium.[37]As this nuclear reaction is part of thealpha processthat produces elements in abundance, sulfur is the 10thmost common element in the universe.
Sulfur, usually as sulfide, is present in many types ofmeteorites.Ordinary chondritescontain on average 2.1% sulfur, andcarbonaceous chondritesmay contain as much as 6.6%. It is normally present astroilite(FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds.[38]The distinctive colors ofJupiter'svolcanicmoonIoare attributed to various forms of molten, solid, and gaseous sulfur.[39]In July 2024, elemental sulfur was confirmed to exist onMarsby surprise, after theCuriosity roverran over and crushed a rock revealing sulfur crystals inside it.[40]
Sulfur is the fifth most common element by mass in the Earth. Elemental sulfur can be found nearhot springsandvolcanicregions in many parts of the world, especially along thePacific Ring of Fire;such volcanic deposits are currently mined in Indonesia, Chile, and Japan. These deposits are polycrystalline, with the largest documented single crystal measuring 22 cm × 16 cm × 11 cm (8.7 in × 6.3 in × 4.3 in).[41]Historically,Sicilywas a major source of sulfur in theIndustrial Revolution.[42]Lakes of molten sulfur up to about 200 m (660 ft) in diameter have been found on the sea floor, associated withsubmarine volcanoes,at depths where the boiling point of water is higher than the melting point of sulfur.[43]
Native sulfur is synthesized byanaerobic bacteriaacting onsulfate mineralssuch asgypsuminsalt domes.[44][45]Significant deposits in salt domes occur along the coast of theGulf of Mexico,and inevaporitesin eastern Europe and western Asia. Native sulfur may be produced by geological processes alone. Fossil-based sulfur deposits from salt domes were once the basis for commercial production in the United States, Russia, Turkmenistan, and Ukraine.[46]Currently, commercial production is still carried out in theOsiekmine in Poland. Such sources are now of secondary commercial importance, and most are no longer worked.
Common naturally occurring sulfur compounds include thesulfide minerals,such aspyrite(iron sulfide),cinnabar(mercury sulfide),galena(lead sulfide),sphalerite(zinc sulfide), andstibnite(antimony sulfide); and thesulfate minerals,such asgypsum(calcium sulfate),alunite(potassium aluminium sulfate), andbarite(barium sulfate). On Earth, just as upon Jupiter's moon Io, elemental sulfur occurs naturally in volcanic emissions, including emissions fromhydrothermal vents.
The main industrial source of sulfur is nowpetroleumandnatural gas.[9]
Compounds
[edit]Commonoxidation statesof sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except thenoble gases.
Electron transfer reactions
[edit]
3)
Sulfur polycations,S2+8,S2+4andS2+16are produced when sulfur is reacted with oxidizing agents in a strongly acidic solution.[47]The colored solutions produced by dissolving sulfur inoleumwere first reported as early as 1804 by C. F. Bucholz, but the cause of the color and the structure of the polycations involved was only determined in the late 1960s.S2+8is deep blue,S2+4is yellow andS2+16is red.[13]
Reduction of sulfur gives variouspolysulfideswith the formulaS2−
x,many of which have been obtained in crystalline form. Illustrative is the production ofsodium tetrasulfide:
Some of these dianions dissociate to giveradical anions,such asS−3gives the blue color of the rocklapis lazuli.

This reaction highlights a distinctive property of sulfur: its ability tocatenate(bind to itself by formation of chains).Protonationof these polysulfide anions produces thepolysulfanes,H2Sx,wherex= 2, 3, and 4.[49]Ultimately, reduction of sulfur produces sulfide salts:
The interconversion of these species is exploited in thesodium–sulfur battery.
Hydrogenation
[edit]Treatment of sulfur with hydrogen giveshydrogen sulfide.When dissolved in water, hydrogen sulfide is mildly acidic:[7]
Hydrogen sulfide gas and the hydrosulfide anion are extremely toxic to mammals, due to their inhibition of the oxygen-carrying capacity ofhemoglobinand certaincytochromesin a manner analogous tocyanideandazide(see below, underprecautions).
Combustion
[edit]The two principal sulfur oxides are obtained by burning sulfur:
Many other sulfur oxides are observed including thesulfur-rich oxidesincludesulfur monoxide,disulfur monoxide,disulfur dioxides, andhigher oxidescontaining peroxo groups.
Halogenation
[edit]Sulfur reacts withfluorineto give the highly reactivesulfur tetrafluorideand the highly inertsulfur hexafluoride.[50]Whereas fluorine gives S(IV) and S(VI) compounds, chlorine gives S(II) and S(I) derivatives. Thus,sulfur dichloride,disulfur dichloride,and higher chlorosulfanes arise from the chlorination of sulfur.Sulfuryl chlorideandchlorosulfuric acidare derivatives of sulfuric acid;thionyl chloride(SOCl2) is a common reagent inorganic synthesis.[51]Bromine also oxidizes sulfur to formsulfur dibromideanddisulfur dibromide.[51]
Pseudohalides
[edit]Sulfur oxidizescyanideandsulfiteto givethiocyanateandthiosulfate,respectively.
Metal sulfides
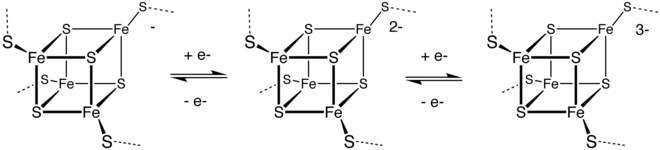
[edit]Sulfur reacts with many metals. Electropositive metals give polysulfide salts. Copper, zinc, and silver are attacked by sulfur; seetarnishing.Although manymetal sulfidesare known, most are prepared by high temperature reactions of the elements.[52]Geoscientists also study the isotopes of metal sulfides in rocks and sediment to study environmental conditions in the Earth's past.[53]
Organic compounds
[edit]- Illustrative organosulfur compounds
-
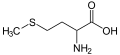
(L)-cysteine,anamino acidcontaining a thiol group
-
Methionine,an amino acid containing a thioether
-
Thiamineor vitamin B1
-
Biotinor vitamin B7
-
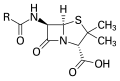
Penicillin,an antibiotic ( "R" is the variable group)
-
Allicin,a chemical compound in garlic
-
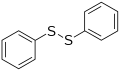
Diphenyl disulfide,a representative disulfide
-
Dibenzothiophene,a component of crude oil
-
Perfluorooctanesulfonic acid(PFOS), a surfactant
Some of the main classes of sulfur-containing organic compounds include the following:[54]
- Thiolsor mercaptans (so called because they capture mercury aschelators) are the sulfur analogs ofalcohols;treatment of thiols with base givesthiolateions.
- Thioethersare the sulfur analogs ofethers.
- Sulfoniumions have three groups attached to a cationic sulfur center.Dimethylsulfoniopropionate(DMSP) is one such compound, important in the marine organicsulfur cycle.
- Sulfoxidesandsulfonesare thioethers with one and two oxygen atoms attached to the sulfur atom, respectively. The simplest sulfoxide,dimethyl sulfoxide,is a common solvent; a common sulfone issulfolane.
- Sulfonic acidsare used in many detergents.
Compounds with carbon–sulfur multiple bonds are uncommon, an exception beingcarbon disulfide,a volatile colorless liquid that is structurally similar to carbon dioxide. It is used as a reagent to make the polymerrayonand many organosulfur compounds. Unlikecarbon monoxide,carbon monosulfideis stable only as an extremely dilute gas, found between solar systems.[55]
Organosulfur compounds are responsible for some of the unpleasant odors of decaying organic matter. They are widely known as theodorantin domestic natural gas, garlic odor, and skunk spray, as well as a component ofbad breathodor. Not all organic sulfur compounds smell unpleasant at all concentrations: the sulfur-containingmonoterpenoidgrapefruit mercaptanin small concentrations is the characteristic scent of grapefruit, but has a generic thiol odor at larger concentrations.Sulfur mustard,a potentvesicant,wasused in World War Ias a disabling agent.[56]
Sulfur–sulfur bonds are a structural component used to stiffen rubber, similar to the disulfide bridges that rigidify proteins (see biological below). In the most common type of industrial "curing" or hardening and strengthening of naturalrubber,elemental sulfur is heated with the rubber to the point that chemical reactions formdisulfidebridges betweenisopreneunits of the polymer. This process, patented in 1843,[citation needed]made rubber a major industrial product, especially in automobile tires. Because of the heat and sulfur, the process was namedvulcanization,after the Roman god of the forge andvolcanism.
History
[edit]Antiquity
[edit]
Being abundantly available in native form, sulfur was known in ancient times and is referred to in theTorah(Genesis).English translations of the Christian Biblecommonly referred to burning sulfur as "brimstone", giving rise to the term "fire-and-brimstone"sermons,in which listeners are reminded of the fate ofeternal damnationthat await the unbelieving and unrepentant. It is from this part of the Bible[57]thatHellis implied to "smell of sulfur" (likely due to its association with volcanic activity). According to theEbers Papyrus,a sulfur ointment was used in ancientEgyptto treat granular eyelids. Sulfur was used forfumigationin preclassicalGreece;[58]this is mentioned in theOdyssey.[59]Pliny the Elderdiscusses sulfur in book 35 of hisNatural History,saying that its best-known source is the island ofMelos.He mentions its use for fumigation, medicine, and bleaching cloth.[60]
A natural form of sulfur known asshiliuhuang(Thạch lưu hoàng) was known in China since the 6th century BC and found inHanzhong.[61]By the 3rd century, the Chinese had discovered that sulfur could be extracted frompyrite.[61]ChineseDaoistswere interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found intraditional Chinese medicine.[61]TheWujing Zongyaoof 1044 AD described various formulas for Chineseblack powder,which is a mixture ofpotassium nitrate(KNO
3),charcoal,and sulfur.[62]
Indian alchemists, practitioners of the "science of chemicals" (Sanskrit:रसशास्त्र,romanized:rasaśāstra), wrote extensively about the use of sulfur in alchemical operations with mercury, from the eighth century AD onwards.[64]In therasaśāstratradition, sulfur is called "the smelly" (गन्धक,gandhaka).
EarlyEuropeanalchemistsgave sulfur a uniquealchemical symbol,a triangle atop a cross (🜍). (This is sometimes confused with the astronomical crossed-spear symbol ⚴ for2 Pallas.) The variation known as brimstone has a symbol combining atwo-barred crossatop alemniscate(🜏). In traditional skin treatment, elemental sulfur was used (mainly in creams) to alleviate such conditions asscabies,ringworm,psoriasis,eczema,andacne.The mechanism of action is unknown—though elemental sulfur does oxidize slowly to sulfurous acid, which is (through the action ofsulfite) a mild reducing and antibacterial agent.[65][66][67]
Modern times
[edit]
Sulfur appears in a column of fixed (non-acidic)alkaliin a chemical table of 1718.[69]Antoine Lavoisierused sulfur in combustion experiments, writing of some of these in 1777.[70]
Sulfur deposits inSicilywere the dominant source for more than a century. By the late 18th century, about 2,000 tonnes per year of sulfur were imported intoMarseille,France, for the production ofsulfuric acidfor use in theLeblanc process.InindustrializingBritain, with the repeal oftariffson salt in 1824, demand for sulfur from Sicily surged upward. The increasing British control and exploitation of the mining, refining, and transportation of the sulfur, coupled with the failure of this lucrative export to transform Sicily's backward and impoverished economy, led to theSulfur Crisis of 1840,whenKing Ferdinand IIgave a monopoly of the sulfur industry to a French firm, violating an earlier 1816 trade agreement with Britain. A peaceful solution was eventually negotiated by France.[71][72]
In 1867, elemental sulfur was discovered in underground deposits inLouisianaandTexas.The highly successfulFrasch processwas developed to extract this resource.[73]
In the late 18th century,furnituremakers used molten sulfur to producedecorative inlays.[74]Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points. Pure powdered sulfur was used as a medicinal tonic and laxative.[46]
With the advent of thecontact process,the majority of sulfur today is used to make sulfuric acid for a wide range of uses, particularly fertilizer.[75]
In recent times, the main source of sulfur has becomepetroleumandnatural gas.This is due to the requirement to remove sulfur from fuels in order to preventacid rain,and has resulted in a surplus of sulfur.[9]
Spelling and etymology
[edit]Sulfuris derived from the Latin wordsulpur,which wasHellenizedtosulphurin the erroneous belief that the Latin word came from Greek. This spelling was later reinterpreted as representing an /f/ sound and resulted in the spellingsulfur,which appears in Latin toward the end of theClassical period.The true Ancient Greek word for sulfur,θεῖον,theîon(from earlierθέειον,théeion), is the source of the international chemical prefixthio-.The Modern Standard Greek word for sulfur is θείο,theío.
In 12th-centuryAnglo-French,it wassulfre.In the 14th century, the erroneously Hellenized Latin-ph-was restored in Middle Englishsulphre.By the 15th century, both full Latin spelling variantssulfurandsulphurbecame common in English. The parallelf~phspellings continued in Britain until the 19th century, when the word was standardized assulphur.[76]On the other hand,sulfurwas the form chosen in the United States, whereas Canada uses both.
TheIUPACadopted the spellingsulfurin 1990[77][78]as did the Nomenclature Committee of theRoyal Society of Chemistryin 1992, restoring the spellingsulfurto Britain.[79]Oxford Dictionariesnote that "in chemistry and other technical uses... the-f-spelling is now the standard form for this and related words in British as well as US contexts, and is increasingly used in general contexts as well. "[80]
Production
[edit]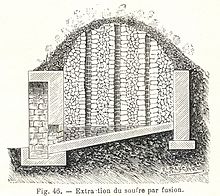

Sulfur may be found by itself and historically was usually obtained in this form;pyritehas also been a source of sulfur.[81]In volcanic regions inSicily,in ancient times, it was found on the surface of the Earth, and the "Sicilian process"was used: sulfur deposits were piled and stacked in brick kilns built on sloping hillsides, with airspaces between them. Then, some sulfur was pulverized, spread over the stacked ore and ignited, causing the free sulfur to melt down the hills. Eventually the surface-borne deposits played out, and miners excavated veins that ultimately dotted the Sicilian landscape with labyrinthine mines. Mining was unmechanized and labor-intensive, with pickmen freeing the ore from the rock, and mine-boys orcarusicarrying baskets of ore to the surface, often through a mile or more of tunnels. Once the ore was at the surface, it was reduced and extracted in smelting ovens. The conditions inSicilian sulfur mineswere horrific, promptingBooker T. Washingtonto write "I am not prepared just now to say to what extent I believe in a physical hell in the next world, but a sulfur mine in Sicily is about the nearest thing to hell that I expect to see in this life."[82]Sulfur is still mined from surface deposits in poorer nations with volcanoes, such asIndonesia,and worker conditions have not improved much since Booker T. Washington's days.[83]
Elemental sulfur was extracted fromsalt domes(in which it sometimes occurs in nearly pure form) until the late 20th century. Sulfur is now produced as a side product of other industrial processes such as in oil refining, in which sulfur is undesired. As a mineral, native sulfur under salt domes is thought to be a fossil mineral resource, produced by the action of anaerobic bacteria on sulfate deposits. It was removed from such salt-dome mines mainly by theFrasch process.[46]In this method, superheated water was pumped into a native sulfur deposit to melt the sulfur, and then compressed air returned the 99.5% pure melted product to the surface. Throughout the 20th century this procedure produced elemental sulfur that required no further purification. Due to a limited number of such sulfur deposits and the high cost of working them, this process for mining sulfur has not been employed in a major way anywhere in the world since 2002.[84][85]

Today, sulfur is produced from petroleum,natural gas,and related fossil resources, from which it is obtained mainly ashydrogen sulfide.[9]Organosulfur compounds,undesirable impurities in petroleum, may be upgraded by subjecting them tohydrodesulfurization,which cleaves the C–S bonds:[84][85]
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by theClaus process.This process entails oxidation of some hydrogen sulfide to sulfur dioxide and then thecomproportionationof the two:[84][85]
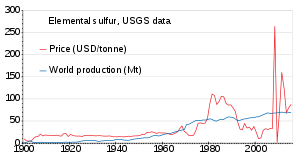
Owing to the high sulfur content of theAthabasca Oil Sands,stockpiles of elemental sulfur from this process now exist throughoutAlberta,Canada.[86]Another way of storing sulfur is as abinderfor concrete, the resulting product having some desirable properties (seesulfur concrete).[87]
The world production of sulfur in 2011 amounted to 69 million tonnes (Mt), with more than 15 countries contributing more than 1 Mt each. Countries producing more than 5 Mt areChina(9.6), theUnited States(8.8),Canada(7.1) andRussia(7.1).[88]Production has been slowly increasing from 1900 to 2010; the price was unstable in the 1980s and around 2010.[89]
Applications
[edit]Sulfuric acid
[edit]Elemental sulfur is used mainly as a precursor to other chemicals. Approximately 85% (1989) is converted tosulfuric acid(H2SO4):

In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical.[89]The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.[46]
Other important sulfur chemistry
[edit]Sulfur reacts directly with methane to givecarbon disulfide,which is used to manufacturecellophaneandrayon.[46]One of the uses of elemental sulfur is invulcanizationof rubber, wherepolysulfidechains crosslink organic polymers. Large quantities ofsulfitesare used tobleachpaperand to preservedried fruit.Manysurfactantsanddetergents(e.g.sodium lauryl sulfate) are sulfate derivatives.Calcium sulfate,gypsum (CaSO4·2H2O) is mined on the scale of 100 milliontonneseach year for use inPortland cementand fertilizers.
When silver-basedphotographywas widespread, sodium and ammoniumthiosulfatewere widely used as "fixing agents". Sulfur is a component ofgunpowder( "black powder" ).
Fertilizer
[edit]Amino acidssynthesized byliving organismssuch asmethionineandcysteinecontainorganosulfurgroups (thioesterandthiolrespectively). Theantioxidantglutathioneprotecting many living organisms againstfree radicalsandoxidative stressalso contains organic sulfur. Somecropssuch asonionandgarlicalso produce differentorganosulfur compoundssuch assyn-propanethial-S-oxideresponsible of lacrymal irritation (onions), ordiallyl disulfideandallicin(garlic).Sulfates,commonly found insoilsandgroundwatersare often a sufficient natural source of sulfur for plants and bacteria.Atmospheric depositionofsulfur dioxide(SO2) is also a common artificial source (coal combustion) of sulfur for the soils. Under normal circumstances, in most agricultural soils, sulfur is not alimiting nutrientfor plants andmicroorganisms(seeLiebig's barrel). However, in some circumstances, soils can be depleted insulfate,e.g. if this later is leached bymeteoric water(rain) or if the requirements in sulfur for some types of crops are high. This explains that sulfur is increasingly recognized and used as a component offertilizers.The most important form of sulfur for fertilizer iscalcium sulfate,commonly found in nature as the mineralgypsum(CaSO4·2H2O). Elemental sulfur ishydrophobic(not soluble in water) and cannot be used directly by plants. Elemental sulfur (ES) is sometimes mixed withbentoniteto amend depleted soils for crops with high requirement in organo-sulfur. Over time,oxidationabioticprocesses withatmosphericoxygenandsoil bacteriacanoxidizeand convert elemental sulfur to soluble derivatives, which can then be used by microorganisms and plants. Sulfur improves the efficiency of other essential plant nutrients, particularlynitrogenand phosphorus.[90]Biologically produced sulfur particles are naturallyhydrophilicdue to abiopolymercoating and are easier to disperse over the land in a spray of diluted slurry, resulting in a faster uptake by plants.
The plants requirement for sulfur equals or exceeds the requirement forphosphorus.It is anessential nutrient for plantgrowth,root noduleformation of legumes, and immunity and defense systems. Sulfur deficiency has become widespread in many countries in Europe.[91][92][93]Because atmospheric inputs of sulfur continue to decrease, the deficit in the sulfur input/output is likely to increase unless sulfur fertilizers are used. Atmospheric inputs of sulfur decrease because of actions taken to limitacid rains.[94][90]
Fungicide and pesticide
[edit]
Elemental sulfur is one of the oldest fungicides andpesticides."Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range ofpowdery mildewdiseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used inorganicallyfarmed apple production against the main diseaseapple scabunder colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster orfrom a dusting plane.Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it watermiscible.[87][95]It has similar applications and is used as afungicideagainstmildewand other mold-related problems with plants and soil.
Elemental sulfur powder is used as an "organic"(i.e.," green ")insecticide(actually anacaricide) againstticksandmites.A common method of application is dusting the clothing or limbs with sulfur powder.
A diluted solution oflime sulfur(made by combiningcalcium hydroxidewith elemental sulfur in water) is used as a dip for pets to destroyringworm (fungus),mange,and otherdermatosesandparasites.
Sulfur candles of almost pure sulfur were burned tofumigatestructures and wine barrels, but are now considered too toxic for residences.
Pharmaceuticals
[edit]Sulfur (specificallyoctasulfur,S8) is used in pharmaceutical skin preparations for the treatment ofacneand other conditions. It acts as akeratolyticagent and also kills bacteria, fungi,scabiesmites, and other parasites.[96]Precipitated sulfur and colloidal sulfur are used, in form oflotions,creams, powders, soaps, and bath additives, for the treatment ofacne vulgaris,acne rosacea,andseborrhoeic dermatitis.[97]
Many drugs contain sulfur.[98]Early examples include antibacterialsulfonamides,known assulfa drugs.A more recent example is mucolyticacetylcysteine.Sulfur is a part of many bacterial defense molecules. Mostβ-lactamantibiotics, including thepenicillins,cephalosporinsandmonobactamscontain sulfur.[54]
Batteries
[edit]Due to their high energy density and the availability of sulfur, there is ongoing research in creating rechargeablelithium–sulfur batteries.Until now, carbonate electrolytes have caused failures in such batteries after a single cycle. In February 2022, researchers atDrexel Universityhave not only created a prototypical battery that lasted 4000 recharge cycles, but also found the first monoclinic gamma sulfur that remained stable below 95 degrees Celsius.[99]
Biological role
[edit]Sulfur is an essential component of all livingcells.It is the eighth most abundant element in the human body by weight,[100]about equal in abundance topotassium,and slightly greater thansodiumandchlorine.[101]A 70 kg (150 lb) human body contains about 140 grams (4.9 oz) of sulfur.[102]The main dietary source of sulfur for humans is sulfur-containing amino-acids,[103]which can be found in plant and animal proteins.[104]
Transferring sulfur between inorganic and biomolecules
[edit]In the 1880s, while studyingBeggiatoa(a bacterium living in a sulfur rich environment),Sergei Winogradskyfound that it oxidizedhydrogen sulfide(H2S) as an energy source, forming intracellular sulfur droplets. Winogradsky referred to this form of metabolism as inorgoxidation (oxidation of inorganic compounds).[105]Another contributor, who continued to study it wasSelman Waksman.[106]Primitive bacteria that live around deep oceanvolcanic ventsoxidize hydrogen sulfide for their nutrition, as discovered byRobert Ballard.[10]
Sulfur oxidizers can use as energy sources reduced sulfur compounds, including hydrogen sulfide, elemental sulfur,sulfite,thiosulfate,and variouspolythionates(e.g.,tetrathionate).[107]They depend on enzymes such assulfur oxygenaseandsulfite oxidaseto oxidize sulfur to sulfate. Somelithotrophscan even use the energy contained in sulfur compounds to produce sugars, a process known aschemosynthesis.Somebacteriaandarchaeause hydrogen sulfide in place of water as theelectron donorin chemosynthesis, a process similar tophotosynthesisthat produces sugars and uses oxygen as theelectron acceptor.Sulfur-based chemosynthesis may be simplifiedly compared with photosynthesis:
There are bacteria combining these two ways of nutrition:green sulfur bacteriaandpurple sulfur bacteria.[108]Also sulfur-oxidizing bacteria can go into symbiosis with larger organisms, enabling the later to use hydrogen sulfide as food to be oxidized. Example: thegiant tube worm.[109]
There aresulfate-reducing bacteria,that, by contrast, "breathe sulfate" instead of oxygen. They use organic compounds or molecular hydrogen as the energy source. They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They can grow on other partially oxidized sulfur compounds (e.g. thiosulfates, thionates, polysulfides, sulfites).
There are studies pointing that many deposits of native sulfur in places that were the bottom ofthe ancient oceanshave biological origin.[110][111][112]These studies indicate that this native sulfur have been obtained through biological activity, but what is responsible for that (sulfur-oxidizing bacteria or sulfate-reducing bacteria) is still unknown for sure.
Sulfur is absorbed byplantsrootsfrom soil assulfateand transported as a phosphate ester. Sulfate is reduced to sulfide via sulfite before it is incorporated intocysteineand other organosulfur compounds.[113]
While the plants' role in transferring sulfur to animals byfood chainsis more or less understood, the role of sulfur bacteria is just getting investigated.[114][115]
Protein and organic metabolites
[edit]In all forms of life, most of the sulfur is contained in twoproteinogenic amino acids(cysteineandmethionine), thus the element is present in allproteinsthat contain these amino acids, as well as in respectivepeptides.[116]Some of the sulfur is comprised in certain metabolites—many of which arecofactors—and sulfated polysaccharides ofconnective tissue(chondroitin sulfates,heparin).
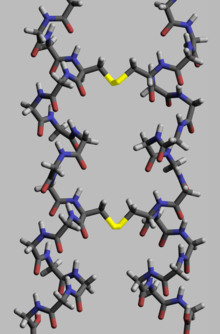
Proteins, to execute theirbiological function,need to have specific space geometry. Formation of this geometry is performed in a process calledprotein folding,and is provided by intra- and inter-molecular bonds. The process has several stages. While at premier stages a polypeptide chain folds due tohydrogen bonds,at later stages folding is provided (apart from hydrogen bonds) bycovalent bondsbetween two sulfur atoms of two cysteine residues (so called disulfide bridges) at different places of a chain (tertiary protein structure) as well as between two cysteine residues in two separated protein subunits (quaternary protein structure). Both structures easily may be seen ininsulin.As thebond energyof a covalent disulfide bridge is higher than the energy of acoordinate bondor hydrophobic interaction, higher disulfide bridges content leads to higher energy needed for proteindenaturation.In general disulfide bonds are necessary in proteins functioning outside cellular space, and they do not change proteins' conformation (geometry), but serve as its stabilizers.[117]Withincytoplasmcysteine residues of proteins are saved in reduced state (i.e. in -SH form) bythioredoxins.[118]
This property manifests in following examples.Lysozymeis stable enough to be applied as a drug.[119]Feathers and hair have relative strength, and consisting in themkeratinis considered indigestible by most organisms. However, there are fungi and bacteria containingkeratinase,and are able to destruct keratin.
Many important cellular enzymes use prosthetic groups ending with -SH moieties to handle reactions involving acyl-containing biochemicals: two common examples from basic metabolism arecoenzyme Aandalpha-lipoic acid.[120]Cysteine-related metaboliteshomocysteineandtaurineare other sulfur-containing amino acids that are similar in structure, but not coded byDNA,and are not part of theprimary structureof proteins, take part in various locations of mammalian physiology.[121][122]Two of the 13 classical vitamins,biotinandthiamine,contain sulfur, and serve as cofactors to several enzymes.[123][124] In intracellular chemistry, sulfur operates as a carrier of reducing hydrogen and its electrons for cellular repair of oxidation. Reducedglutathione,a sulfur-containing tripeptide, is a reducing agent through its sulfhydryl (–SH) moiety derived fromcysteine.
Methanogenesis,the route to most of the world's methane, is a multistep biochemical transformation ofcarbon dioxide.This conversion requires several organosulfur cofactors. These includecoenzyme M,CH3SCH2CH2SO−3,the immediate precursor tomethane.[125]
Metalloproteins and inorganic cofactors
[edit]Metalloproteins—in which the active site is a transition metal ion (or metal-sulfide cluster) often coordinated by sulfur atoms of cysteine residues[126]—are essential components of enzymes involved in electron transfer processes. Examples includeplastocyanin(Cu2+) andnitrous oxide reductase(Cu–S). The function of these enzymes is dependent on the fact that the transition metal ion can undergoredox reactions.Other examples include many zinc proteins,[127]as well asiron–sulfur clusters.Most pervasive are theferrodoxins,which serve as electron shuttles in cells. In bacteria, the importantnitrogenaseenzymes contain an Fe–Mo–S cluster and is acatalystthat performs the important function ofnitrogen fixation,converting atmospheric nitrogen to ammonia that can be used by microorganisms and plants to make proteins, DNA, RNA, alkaloids, and the other organic nitrogen compounds necessary for life.[128]
Sulfur is also present inmolybdenum cofactor.[129]
Sulfate
[edit]Deficiency
[edit]In humansmethionineis anessential amino acid;cysteineis conditionally essential and may be synthesized from non-essentialserine(sulfur donor would be methionine in this case). Dietary deficiency rarely happens in common conditions. Artificial methionine deficiency is attempted to apply in cancer treatment,[130]but the method is still potentially dangerous.[131]
Isolated sulfite oxidase deficiencyis a rare, fatal genetic disease preventing production ofsulfite oxidase,needed to metabolize sulfites to sulfates.[132]
Precautions
[edit]| Hazards | |
|---|---|
| GHSlabelling: | |
 
| |
| Warning | |
| H315[133] | |
| NFPA 704(fire diamond) | |

Though elemental sulfur is only minimally absorbed through the skin and is of low toxicity to humans, inhalation of sulfur dust or contact with eyes or skin may cause irritation. Excessive ingestion of sulfur can cause a burning sensation or diarrhea,[135]and cases of life-threatening metabolic acidosis have been reported after patients deliberately consumed sulfur as a folk remedy.[136][137]
Toxicity of sulfur compounds
[edit]When sulfur burns in air, it producessulfur dioxide.In water, this gas produces sulfurous acid and sulfites; sulfites are antioxidants that inhibit growth of aerobic bacteria and a usefulfood additivein small amounts. At high concentrations these acids harm thelungs,eyes,or othertissues.[138]In organisms without lungs such as insects, sulfite in high concentration preventsrespiration.[139]
Sulfur trioxide(made by catalysis from sulfur dioxide) andsulfuric acidare similarly highly acidic and corrosive in the presence of water. Concentrated sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic tissue.[140]
The burning ofcoaland/orpetroleumby industry andpower plantsgenerates sulfur dioxide (SO2) that reacts with atmospheric water and oxygen to producesulfurous acid(H2SO3).[141]These acids are components ofacid rain,lowering thepHofsoiland freshwater bodies, sometimes resulting in substantial damage to theenvironmentandchemical weatheringof statues and structures. Fuel standards increasingly require that fuel producers extract sulfur fromfossil fuelsto prevent acid rain formation. This extracted and refined sulfur represents a large portion of sulfur production. In coal-fired power plants,flue gasesare sometimes purified. More modern power plants that usesynthesis gasextract the sulfur before they burn the gas.
Hydrogen sulfideis about one-half astoxicashydrogen cyanide,and intoxicates by the same mechanism (inhibition of the respiratory enzymecytochrome oxidase),[142]though hydrogen sulfide is less likely to cause sudden poisonings from small inhaled amounts (near itspermissible exposure limit(PEL) of 20 ppm) because of its disagreeable odor.[143]However, its presence in ambient air at concentration over 100–150 ppm quickly deadens the sense of smell,[144]and a victim may breathe increasing quantities without noticing until severe symptoms cause death. Dissolvedsulfideandhydrosulfidesalts are toxic by the same mechanism.
Notes
[edit]- ^But impure samples have an odor similar to that ofmatches.A strong odor called "smell of sulfur" actually is given off by several sulfur compounds, such ashydrogen sulfideandorganosulfurcompounds.
- ^A figure of sulfur's melting point in 115.21°C had been determined by two laboratories of the US Department of Energy (Jefferson Lab and Los Alamos National Lab).[12] Greenwood and Earnshaw say that at fast heating for microcrystalline α-S8the melting point in 115.1 °C (239.2 °F) is specified.[7]
- ^Historically, it was a rather difficult task to find the exact melting point of sulfur.[15]When heated sluggishly, the factual melting point may lie within the range from 114.6 °C (238.3 °F) or even lower, to 120.4 °C (248.7 °F)[7](factors, that interfere a definite melting point, is apolymerlike natureof sulfur[16]and a large number of allotropes.[17]) Melting point may be presented as a temperature interval, depending on the allotrope composition of a sample at a moment of melting.
See also
[edit]- Blue lava
- Stratospheric sulfur aerosols
- Sulfur assimilation
- Sulfur isotope biogeochemistry
- Ultra-low sulfur diesel
References
[edit]- ^"Standard Atomic Weights: Sulfur".CIAAW.2009.
- ^Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022)."Standard atomic weights of the elements 2021 (IUPAC Technical Report)".Pure and Applied Chemistry.doi:10.1515/pac-2019-0603.ISSN1365-3075.
- ^abArblaster, John W. (2018).Selected Values of the Crystallographic Properties of Elements.Materials Park, Ohio: ASM International.ISBN978-1-62708-155-9.
- ^Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds".CRC Handbook of Chemistry and Physics(PDF)(86th ed.). Boca Raton (FL): CRC Press.ISBN0-8493-0486-5.
- ^Weast, Robert (1984).CRC, Handbook of Chemistry and Physics.Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110.ISBN0-8493-0464-4.
- ^"Sulfur History".Georgiagulfsulfur.com.Retrieved12 February2022.
- ^abcdeGreenwood, N. N.; Earnshaw, A. (1997).Chemistry of the Elements(2nd ed.). Oxford: Butterworth-Heinemann.ISBN0-7506-3365-4.
- ^Chisholm, Hugh,ed. (1911)..Encyclopædia Britannica.Vol. 4 (11th ed.). Cambridge University Press. p. 571.
- ^abcdLaurence Knight (19 July 2014)."Sulphur surplus: Up to our necks in a diabolical element".BBC.
- ^ab"Sulfur".Elements.BBC. 11 October 2014..Downloadhere.
- ^Rettig, S. J.; Trotter, J. (15 December 1987)."Refinement of the structure of orthorhombic sulfur, α-S8"(PDF).Acta Crystallographica Section C.43(12): 2260–2262.Bibcode:1987AcCrC..43.2260R.doi:10.1107/S0108270187088152.ISSN0108-2701.
- ^"Sulfur | S (Element) - PubChem".pubchem.ncbi.nlm.nih.gov.Retrieved15 April2024.
- ^abcGreenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.pp. 645–665.ISBN978-0-08-037941-8.
- ^Poling, Bruce E.; Prausnitz, John M.; O'Connell, John P. (27 November 2000).The Properties of Gases and Liquids 5E.McGraw Hill Professional.ISBN978-0-07-149999-6.
- ^"7.5: Changes of State".Chemistry LibreTexts.3 October 2013.Retrieved15 April2024.
- ^Kozhevnikov, V. F.; Payne, W. B.; Olson, J. K.; McDonald, C. L.; Inglefield, C. E. (15 October 2004)."Physical properties of sulfur near the polymerization transition".The Journal of Chemical Physics.121(15): 7379–7386.arXiv:physics/0405012.Bibcode:2004JChPh.121.7379K.doi:10.1063/1.1794031.ISSN0021-9606.PMID15473808.
- ^Inostroza, Manuel; Fernandez, Bárbara; Aguilera, Felipe; Layana, Susana; Walter, Thomas R.; Zimmer, Martin; Rodríguez-Díaz, Augusto; Oelze, Marcus (2023)."Physical and chemical characteristics of active sulfur flows observed at Lastarria volcano (northern Chile) in January 2019".Frontiers in Earth Science.11.Bibcode:2023FrEaS..1197363I.doi:10.3389/feart.2023.1197363.ISSN2296-6463.
- ^Tucker, Roy P. (1 January 1929)."Notes on the Sublimation of Sulfur between 25° and 50°C".Industrial & Engineering Chemistry.21(1): 44–47.doi:10.1021/ie50229a014.ISSN0019-7866.
- ^Maldonado-Zagal, S. B.; Boden, P. J. (1 January 1982)."Hydrolysis of Elemental Sulphur in Water and its Effect on the Corrosion of Mild Steel".British Corrosion Journal.17(3): 116–120.doi:10.1179/000705982798274336.ISSN0007-0599.Retrieved23 June2022.
- ^Munson, Ronald A. (February 1968)."The synthesis of iridium disulfide and nickel diarsenide having the pyrite structure"(PDF).Inorganic Chemistry.7(2): 389–390.doi:10.1021/ic50060a047.Archived fromthe original(PDF)on 12 April 2019.Retrieved19 January2019.
- ^Egon Wiberg; Nils Wiberg (2001).Inorganic Chemistry.Academic Press. pp. 513–.ISBN978-0-12-352651-9.
- ^Steudel, Ralf; Eckert, Bodo (2003).Solid Sulfur Allotropes Sulfur Allotropes.Topics in Current Chemistry. Vol. 230. pp. 1–80.doi:10.1007/b12110.ISBN978-3-540-40191-9.
- ^Steudel, R. (1982). "Homocyclic sulfur molecules".Inorganic Ring Systems.Topics in Current Chemistry. Vol. 102. pp. 149–176.doi:10.1007/3-540-11345-2_10.ISBN978-3-540-11345-4.
- ^Tebbe, Fred N.; Wasserman, E.; Peet, William G.; Vatvars, Arturs; Hayman, Alan C. (1982). "Composition of Elemental Sulfur in Solution: Equilibrium ofS
6,S7,and S8at Ambient Temperatures ".Journal of the American Chemical Society.104(18): 4971–4972.doi:10.1021/ja00382a050. - ^Meyer, Beat (1964). "Solid Allotropes of Sulfur".Chemical Reviews.64(4): 429–451.doi:10.1021/cr60230a004.
- ^Meyer, Beat (1976). "Elemental sulfur".Chemical Reviews.76(3): 367–388.doi:10.1021/cr60301a003.
- ^Sulfur.Commission on Isotopic Abundances and Atomic Weights
- ^Haynes, William M., ed. (2011).CRC Handbook of Chemistry and Physics(92nd ed.). Boca Raton, FL:CRC Press.p. 1.14.ISBN1-4398-5511-0.
- ^"Searching for the Origins of Presolar Grains".Energy.gov.Retrieved4 February2023.
- ^Paytan, Adina; Yao, Weiqi; Faul, Kristina; Gray, E.T. (2020)."Sulfur Isotope Stratigraphy".Geologic Time Scale.pp. 259–278.doi:10.1016/B978-0-12-824360-2.00009-7.ISBN9780128243602.
- ^"NASA Astrobiology".astrobiology.nasa.gov.Retrieved4 February2023.
- ^Goldhaber, M.B.; Kaplan, I.R. (April 1980)."Mechanisms of sulfur incorporation and isotope fractionation during early diagenesis in sediments of the gulf of California".Marine Chemistry.9(2): 95–143.Bibcode:1980MarCh...9...95G.doi:10.1016/0304-4203(80)90063-8.
- ^Sim, Min Sub; Bosak, Tanja; Ono, Shuhei (July 2011)."Large Sulfur Isotope Fractionation Does Not Require Disproportionation".Science.333(6038): 74–77.Bibcode:2011Sci...333...74S.doi:10.1126/science.1205103.ISSN0036-8075.PMID21719675.S2CID1248182.
- ^Tsang, Man-Yin; Böttcher, Michael Ernst; Wortmann, Ulrich Georg (August 2023)."Estimating the effect of elemental sulfur disproportionation on the sulfur-isotope signatures in sediments".Chemical Geology.632:121533.doi:10.1016/j.chemgeo.2023.121533.S2CID258600480.
- ^Kim, Ik Soo; Kwak, Seung Im; Park, Ul Jae; Bang, Hong Sik; Han, Hyun Soo (1 July 2005).Production of Sulfur-35 by the Cation Exchange Process.2005 autumn meeting of the KNS, Busan (Korea, Republic of), 27–28 Oct 2005.
- ^"Sulfur-35 (35 S) safety information and specific handling precautions"(PDF).Yale Environmental Health & Safety.
- ^Cameron, A. G. W. (1957)."Stellar Evolution, Nuclear Astrophysics, and Nucleogenesis"(PDF).CRL-41.
- ^Mason, B. (1962).Meteorites.New York: John Wiley & Sons. p.160.ISBN978-0-908678-84-6.
- ^Lopes, Rosaly M. C.;Williams, David A. (2005). "Io after Galileo".Reports on Progress in Physics.68(2): 303–340.Bibcode:2005RPPh...68..303L.doi:10.1088/0034-4885/68/2/R02.S2CID44208045.
- ^Strickland, Ashley (20 July 2024)."NASA's Curiosity rover makes its 'most unexpected' find on Mars".CNN.Retrieved21 July2024.
- ^Rickwood, P. C. (1981)."The largest crystals"(PDF).American Mineralogist.66:885–907.
- ^Kutney, Gerald (2007).Sulfur: history, technology, applications & industry.Toronto: ChemTec. p. 43.ISBN978-1-895198-37-9.OCLC79256100.
- ^de Ronde, C. E. J.; Chadwick, W. W. Jr.; Ditchburn, R. G.; Embley, R. W.; Tunnicliffe, V.; Baker, E. T.; Walker, S. L.; Ferrini, V. L.; Merle, S. M. (2015). "Molten Sulfur Lakes of Intraoceanic Arc Volcanoes".Volcanic Lakes.Springer. pp. 261–288.Bibcode:2015vola.book.....R.doi:10.1007/978-3-642-36833-2.ISBN978-3-642-36832-5.S2CID199492543.
- ^Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1985).Manual of Mineralogy(20th ed.). Wiley. pp. 265–66.ISBN0-471-80580-7.
- ^"Sulphur: Mineral information, data and localities".www.mindat.org.
- ^abcdeNehb, Wolfgang; Vydra, Karel (2006). "Sulfur".Ullmann's Encyclopedia of Industrial Chemistry.Wiley-VCH Verlag.doi:10.1002/14356007.a25_507.pub2.ISBN978-3-527-30673-2.
- ^Shriver, Atkins. Inorganic Chemistry, Fifth Edition. W. H. Freeman and Company, New York, 2010; pp 416
- ^Fujimori, Toshihiko; Morelos-Gómez, Aarón; Zhu, Zhen; Muramatsu, Hiroyuki; Futamura, Ryusuke; Urita, Koki; Terrones, Mauricio; Hayashi, Takuya; Endo, Morinobu; Young Hong, Sang; Chul Choi, Young; Tománek, David; Kaneko, Katsumi (2013)."Conducting linear chains of sulphur inside carbon nanotubes".Nature Communications.4:2162.Bibcode:2013NatCo...4.2162F.doi:10.1038/ncomms3162.PMC3717502.PMID23851903.
- ^Brauer, G., ed. (1963).Handbook of Preparative Inorganic Chemistry.Vol. 1 (2nd ed.). New York: Academic Press. p. 421.
- ^Hasek, W. R. (1961). "1,1,1-Trifluoroheptane".Organic Syntheses.41:104.doi:10.1002/0471264180.os041.28.
- ^abLauss, H.-D.; Steffens, W. "Sulfur Halides".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a25_623.ISBN978-3527306732.
- ^Vaughan, D. J.; Craig, J. R. (1978).Mineral Chemistry of Metal Sulfides.Cambridge: Cambridge University Press.ISBN0-521-21489-0.
- ^Tsang, Man-Yin; Inagaki, Fumio (29 May 2020)."Microbial Life Deep Under the Seafloor—A Story of Not Giving Up".Frontiers for Young Minds.8:70.doi:10.3389/frym.2020.00070.ISSN2296-6846.
- ^abCremlyn, R. J. (1996).An Introduction to Organosulfur Chemistry.Chichester: John Wiley and Sons.ISBN0-471-95512-4.
- ^Wilson, R. W.;Penzias, A. A.;Wannier, P. G.; Linke, R. A. (15 March 1976)."Isotopic abundances in interstellar carbon monosulfide".Astrophysical Journal.204:L135–L137.Bibcode:1976ApJ...204L.135W.doi:10.1086/182072.
- ^Banoub, Joseph (2011).Detection of Biological Agents for the Prevention of Bioterrorism.NATO Science for Peace and Security Series A: Chemistry and Biology. p. 183.Bibcode:2011dbap.book.....B.doi:10.1007/978-90-481-9815-3.ISBN978-90-481-9815-3.OCLC697506461.
- ^"Sulfur in the Bible (14 instances)".bible.knowing-jesus.com.Retrieved19 May2022.
- ^Rapp, George Robert (4 February 2009).Archaeomineralogy.Springer. p. 242.ISBN978-3-540-78593-4.
- ^Odyssey,book 22, lines 480–495.www.perseus.tufts.edu. Retrieved on 16 August 2012.
- ^Pliny the Elder on science and technology,John F. Healy, Oxford University Press, 1999,ISBN0-19-814687-6,pp. 247–249.
- ^abcZhang, Yunming (1986). "The History of Science Society: Ancient Chinese Sulfur Manufacturing Processes".Isis.77(3): 487.doi:10.1086/354207.S2CID144187385.
- ^Needham, Joseph;Yates, Robin(1994).Science and Civilisation in China, Volume 5: Chemistry and Chemical Technology, Part 6, Military Technology: Missiles and Sieges.Cambridge: Cambridge University Press. p. 120.ISBN9780521327275.OCLC489677531.
- ^Koch, Rudolf (1955).The book of signs: which contains all manner of symbols used from the earliest times to the Middle Ages by primitive peoples and early Christians.New York: Dover Publications.ISBN0-486-20162-7.
- ^White, David Gordon (1996).The Alchemical Body — Siddha Traditions in Medieval India.Chicago: University of Chicago Press. pp. passim.ISBN978-0-226-89499-7.
- ^Lin, A. N.; Reimer, R. J.; Carter, D. M. (1988). "Sulfur revisited".Journal of the American Academy of Dermatology.18(3): 553–558.doi:10.1016/S0190-9622(88)70079-1.PMID2450900.
- ^Maibach, H. I.; Surber, C.; Orkin, M. (1990)."Sulfur revisited".Journal of the American Academy of Dermatology.23(1): 154–156.doi:10.1016/S0190-9622(08)81225-X.PMID2365870.
- ^Gupta, A. K.; Nicol, K. (2004). "The use of sulfur in dermatology".Journal of Drugs in Dermatology.3(4): 427–31.PMID15303787.
- ^Gupta, Aditya K; Nicol, Karyn (July–August 2004)."The Use of Sulfur in Dermatology".Journal of Drugs in Dermatology.3(4): 427–431.PMID15303787.
- ^Donovan, Arthur (1996).Antoine Lavoisier: Science, Administration and Revolution.Cambridge University Press. p. 66.ISBN978-0-521-56672-8.
- ^Poirier, Jean-Pierre (1998).Lavoisier: Chemist, Biologist, Economist.University of Pennsylvania Press. pp. 107–8.ISBN978-0-8122-1649-3.
- ^Riall, Lucy (1998).Sicily and the Unification of Italy: Liberal Policy and Local Power, 1859–1866.Oxford University Press.ISBN9780191542619.Retrieved7 February2013.
- ^Thomson, D. W. (April 1995). "Prelude to the Sulphur War of 1840: The Neapolitan Perspective".European History Quarterly.25(2): 163–180.doi:10.1177/026569149502500201.S2CID145807900.
- ^Botsch, Walter (2001). "Chemiker, Techniker, Unternehmer: Zum 150. Geburtstag von Hermann Frasch".Chemie in unserer Zeit(in German).35(5): 324–331.doi:10.1002/1521-3781(200110)35:5<324::AID-CIUZ324>3.0.CO;2-9.
- ^Mass, Jennifer L; Anderson, Mark J (2003). "Pennsylvania German sulfur-inlaid furniture: characterization, reproduction, and ageing phenomena of the inlays".Measurement Science and Technology.14(9): 1598.doi:10.1088/0957-0233/14/9/311.ISSN0957-0233.S2CID250882259.
- ^Kogel, Jessica (2006).Industrial minerals & rocks: commodities, markets, and uses(7th ed.). Colorado: Littleton. p. 935.ISBN978-0-87335-233-8.OCLC62805047.
- ^"sulphur".Oxford English Dictionary(Online ed.).Oxford University Press.(Subscription orparticipating institution membershiprequired.)
- ^"So long sulphur".Nature Chemistry.1(5): 333. 4 August 2009.Bibcode:2009NatCh...1Q.333..doi:10.1038/nchem.301.PMID21378874.
- ^International Union of Pure and Applied Chemistry: Inorganic Chemistry Division, Commission on Nomenclature of Inorganic Chemistry (1990).Nomenclature of Inorganic Chemistry, (Recommendations 1990).Oxford, UK: Blackwell Scientific Publications. pp. 39, 40, 41, 240, 247.
- ^McNaught, Alan (1991). "Journal style update".The Analyst.116(11): 1094.Bibcode:1991Ana...116.1094M.doi:10.1039/AN9911601094.
- ^"sulphur – definition of sulphur in English".Oxford Dictionaries.Archived fromthe originalon 20 November 2016.Retrieved19 November2016.
- ^Riegel, Emil; Kent, James (2007).Kent and Riegel's Handbook of Industrial Chemistry and Biotechnology.Vol. 1. Springer. p. 1171.Bibcode:2007karh.book.......ISBN978-0-387-27842-1.OCLC74650396.
- ^Washington, Booker T. (1912).The Man Farthest Down: A Record of Observation and Study in Europe.Doubleday, Page. p. 214.
- ^McElvaney, Kevin (25 February 2015)."The Men Who Mine Volcanos".The Atlantic.Retrieved26 February2015.
- ^abcEow, John S. (2002). "Recovery of sulfur from sour acid gas: A review of the technology".Environmental Progress.21(3): 143–162.Bibcode:2002EnvPr..21..143E.doi:10.1002/ep.670210312.
- ^abcSchreiner, Bernhard (2008). "Der Claus-Prozess. Reich an Jahren und bedeutender denn je".Chemie in unserer Zeit.42(6): 378–392.doi:10.1002/ciuz.200800461.
- ^Hyndman, A. W.; Liu, J. K.; Denney, D. W. (1982). "Sulfur Recovery from Oil Sands".Sulfur: New Sources and Uses.ACS Symposium Series. Vol. 183. pp. 69–82.doi:10.1021/bk-1982-0183.ch005.ISBN978-0-8412-0713-4.
- ^abMohamed, Abdel-Mohsen Onsy; El-Gamal, Maisa M. (2010).Sulfur concrete for the construction industry: a sustainable development approach.Fort Lauderdale:J. Ross. pp. 104–105, 109.ISBN978-1-60427-005-1.OCLC531718953.
- ^Apodaca, Lori E. (2012)"Sulfur".Mineral Commodity Summaries.United States Geological Survey.
- ^abApodaca, Lori E."Mineral Yearbook 2010: Sulfur"(PDF).United States Geological Survey.
- ^ab"FAQ – The Sulphur Institute".sulphurinstitute.org.The Sulphur Institute.2020.Retrieved27 February2020.
- ^Zhao, F.; Hawkesford, M. J.; McGrath, S. P. (1999). "Sulphur Assimilation and Effects on Yield and Quality of Wheat".Journal of Cereal Science.30(1): 1–17.doi:10.1006/jcrs.1998.0241.
- ^Blake-Kalff, M. M. A. (2000). "Diagnosing sulfur deficiency in field-grown oilseed rape (Brassica napus L.) and wheat (Triticum aestivum L.)".Plant and Soil.225(1/2): 95–107.doi:10.1023/A:1026503812267.S2CID44208638.
- ^Ceccotti, S. P. (1996). "Plant nutrient sulphur-a review of nutrient balance, environmental impact and fertilizers".Fertilizer Research.43(1–3): 117–125.doi:10.1007/BF00747690.S2CID42207099.
- ^Glossary,United States:NASA Earth Observatory,acid rain,archivedfrom the original on 13 December 2011,retrieved15 February2013
- ^Every, Richard L.; et al. (20 August 1968)."Method for Preparation of Wettable Sulfur"(PDF).Retrieved20 May2010.
- ^Hagers Handbuch der Pharmazeutischen Praxis(in German). Vol. 6B (4th ed.). Berlin–Heidelberg–New York: Springer. 1978. pp. 672–9.ISBN978-3-540-07738-1.
- ^Arzneibuch-Kommentar. Wissenschaftliche Erläuterungen zum Europäischen Arzneibuch und zum Deutschen Arzneibuch[Pharmacopoeia Commentary. Scientific annotations to the European Pharmacopoeia and the German Pharmacopoeia] (in German) (23rd ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. 2004. MonographieSchwefel zum äußerlichen Gebrauch[MonographSulfur for external use].ISBN978-3-8047-2575-1.
- ^Scott, Kevin A.; Njardarson, Jon T. (2019), Jiang, Xuefeng (ed.),"Analysis of US FDA-Approved Drugs Containing Sulfur Atoms"(PDF),Sulfur Chemistry,Topics in Current Chemistry Collections, Springer International Publishing, pp. 1–34,doi:10.1007/978-3-030-25598-5_1,ISBN978-3-030-25598-5,retrieved8 March2023
- ^Pai, Rahul (10 February 2022)."Stabilization of gamma sulfur at room temperature to enable the use of carbonate electrolyte in Li–S batteries".Communications Chemistry.5(1): 17.doi:10.1038/s42004-022-00626-2.PMC9814344.PMID36697747.S2CID246704531.
- ^"Sulphur and the Human Body".The Sulfur Institute.Retrieved3 April2021.
- ^"What is the body made of?".New Scientist.Archivedfrom the original on 3 November 2021.Retrieved9 November2021.
- ^Helmenstine, Anne (3 February 2019)."Elemental Composition of the Human Body by Mass".ThoughtCo.Archivedfrom the original on 13 April 2021.Retrieved21 November2021.
- ^Parcell, Stephen (February 2002)."Sulfur in human nutrition and applications in medicine".Alternative Medicine Review.7(1): 22–44.ISSN1089-5159.PMID11896744.
- ^Ingenbleek, Yves; Kimura, Hideo (July 2013)."Nutritional essentiality of sulfur in health and disease".Nutrition Reviews.71(7): 413–432.doi:10.1111/nure.12050.ISSN1753-4887.PMID23815141.
- ^Dworkin, Martin (March 2012)."Sergei Winogradsky: a founder of modern microbiology and the first microbial ecologist".FEMS Microbiology Reviews.36(2): 364–379.doi:10.1111/j.1574-6976.2011.00299.x.ISSN1574-6976.PMID22092289.
- ^Waksman, S. A.; Starkey, R. L. (20 January 1923)."On the Growth and Respiration of Sulfur-Oxidizing Bacteria".The Journal of General Physiology.5(3): 285–310.doi:10.1085/jgp.5.3.285.ISSN0022-1295.PMC2140527.PMID19871997.
- ^Pronk JT; Meulenberg R; Hazeu W; Bos P; Kuenen JG (1990)."Oxidation of reduced inorganic sulphur compounds by acidophilic thiobacilli".FEMS Microbiology Letters.75(2–3): 293–306.doi:10.1111/j.1574-6968.1990.tb04103.x.
- ^Frigaard, Niels-Ulrik; Dahl, Christiane (1 January 2008), Poole, Robert K. (ed.),Sulfur Metabolism in Phototrophic Sulfur Bacteria,Advances in Microbial Physiology, vol. 54, Academic Press, pp. 103–200,doi:10.1016/S0065-2911(08)00002-7,ISBN9780123743237,PMID18929068,retrieved17 May2022
- ^Cavanaugh, Colleen M. (1994)."Microbial Symbiosis: Patterns of Diversity in the Marine Environment".American Zoologist.34:79–89.doi:10.1093/icb/34.1.79.
- ^Jones, Galen E.; Starkey, Robert L.; Feely, Herbert W.; Kulp, J. Laurence (22 June 1956)."Biological Origin of Native Sulfur in Salt Domes of Texas and Louisiana".Science.123(3208): 1124–1125.Bibcode:1956Sci...123.1124J.doi:10.1126/science.123.3208.1124.ISSN0036-8075.PMID17793426.
- ^Philip, G.; Wali, A. M. A.; Aref, M. A. M. (1 September 1994)."On the origin of native sulfur deposits in Gebel El Zeit, Gulf of Suez, Egypt".Carbonates and Evaporites.9(2): 223–232.Bibcode:1994CarEv...9..223P.doi:10.1007/BF03175232.ISSN1878-5212.S2CID128827551.
- ^"Petrography and mineralogy of the crystalline limestone of Fatha Formation from Mishraq area, Iraq".ResearchGate.Retrieved15 April2022.
- ^Heldt, Hans-Walter (1996).Pflanzenbiochemie(in German). Heidelberg: Spektrum Akademischer Verlag. pp. 321–333.ISBN978-3-8274-0103-8.
- ^Kuenen, J. G.; Beudeker, R. F. (13 September 1982)."Microbiology of thiobacilli and other sulphur-oxidizing autotrophs, mixotrophs and heterotrophs".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.298(1093): 473–497.Bibcode:1982RSPTB.298..473K.doi:10.1098/rstb.1982.0093.ISSN0962-8436.PMID6127737.
- ^Wasmund, Kenneth; Mußmann, Marc; Loy, Alexander (August 2017)."The life sulfuric: microbial ecology of sulfur cycling in marine sediments: Microbial sulfur cycling in marine sediments".Environmental Microbiology Reports.9(4): 323–344.doi:10.1111/1758-2229.12538.PMC5573963.PMID28419734.
- ^Gutiérrez-Preciado, A.; Romero, H.; Peimbert, M. (2010)."An Evolutionary Perspective on Amino Acids".Nature Education.3(9): 29.
- ^Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002).Molecular Biology of the Cell. 4th edition.New York: Garland Science.ISBN978-0-8153-3218-3.
- ^Arnér, Elias S. J.; Holmgren, Arne (25 December 2001)."Physiological functions of thioredoxin and thioredoxin reductase: Thioredoxin and thioredoxin reductase".European Journal of Biochemistry.267(20): 6102–6109.doi:10.1046/j.1432-1327.2000.01701.x.PMID11012661.
- ^"Lysozyme".Drugs.com.Retrieved19 May2022.
- ^Nelson, D. L.; Cox, M. M. (2000).Lehninger, Principles of Biochemistry(3rd ed.). New York: Worth Publishing.ISBN978-1-57259-153-0.
- ^Selhub, J. (1 July 1999)."Homocysteine metabolism".Annual Review of Nutrition.19(1): 217–246.doi:10.1146/annurev.nutr.19.1.217.ISSN0199-9885.PMID10448523.
- ^Huxtable, R. J. (1 January 1992)."Physiological actions of taurine".Physiological Reviews.72(1): 101–163.doi:10.1152/physrev.1992.72.1.101.ISSN0031-9333.PMID1731369.
- ^"The Function of Biotin".www.chem.uwec.edu.Retrieved10 June2022.
- ^Edwards, Katie A."Thiamine Biochemistry".thiamine.dnr.cornell.edu.Retrieved10 June2022.
- ^Thauer, R. K. (1998)."Biochemistry of methanogenesis: a tribute to Marjory Stephenson:1998 Marjory Stephenson Prize Lecture".Microbiology.144(9): 2377–2406.doi:10.1099/00221287-144-9-2377.PMID9782487.
- ^Pace, Nicholas J.; Weerapana, Eranthie (17 April 2014)."Zinc-binding cysteines: diverse functions and structural motifs".Biomolecules.4(2): 419–434.doi:10.3390/biom4020419.ISSN2218-273X.PMC4101490.PMID24970223.
- ^Giles, Niroshini M; Watts, Aaron B; Giles, Gregory I; Fry, Fiona H; Littlechild, Jennifer A; Jacob, Claus (1 August 2003)."Metal and Redox Modulation of Cysteine Protein Function".Chemistry & Biology.10(8): 677–693.doi:10.1016/S1074-5521(03)00174-1.ISSN1074-5521.PMID12954327.
- ^Lippard, S. J.; Berg, J. M. (1994).Principles of Bioinorganic Chemistry.University Science Books.ISBN978-0-935702-73-6.
- ^Schwarz, Günter; Mendel, Ralf R. (2006). "Molybdenum cofactor biosynthesis and molybdenum enzymes".Annual Review of Plant Biology.57(1): 623–647.doi:10.1146/annurev.arplant.57.032905.105437.ISSN1543-5008.PMID16669776.
- ^Mladenović, Dušan; Radosavljević, Tatjana; Hrnčić, Dragan; Rasic-Markovic, Aleksandra; Stanojlović, Olivera (26 July 2019)."The effects of dietary methionine restriction on the function and metabolic reprogramming in the liver and brain – implications for longevity".Reviews in the Neurosciences.30(6): 581–593.doi:10.1515/revneuro-2018-0073(inactive 27 August 2024).ISSN2191-0200.PMID30817309.S2CID73470156.
{{cite journal}}:CS1 maint: DOI inactive as of August 2024 (link) - ^Binz, Regina L.; Sadhukhan, Ratan; Miousse, Isabelle R.; Garg, Sarita; Koturbash, Igor; Zhou, Daohong; Hauer-Jensen, Martin; Pathak, Rupak (27 February 2021)."Dietary Methionine Deficiency Enhances Genetic Instability in Murine Immune Cells".International Journal of Molecular Sciences.22(5): 2378.doi:10.3390/ijms22052378.ISSN1422-0067.PMC7956689.PMID33673497.
- ^Karakas, Erkan; Kisker, Caroline (18 October 2005)."Structural analysis of missense mutations causing isolated sulfite oxidase deficiency".Dalton Transactions(21): 3459–3463.doi:10.1039/B505789M.ISSN1477-9234.PMID16234925.
- ^"Sulfur 84683".S.
- ^"Chemical Datasheet. SULFUR, MOLTEN".
- ^"Sulfur General Fact Sheet".npic.orst.edu.Retrieved2 September2022.
- ^Schwartz, Steven M.; Carroll, Hugh M.; Scharschmidt, Linda A. (1 July 1986)."Sublimed (Inorganic) Sulfur Ingestion: A Cause of Life-Threatening Metabolic Acidosis With a High Anion Gap".Archives of Internal Medicine.146(7): 1437–1438.doi:10.1001/archinte.1986.00360190229034.ISSN0003-9926.PMID3718141.
- ^Blum, J. Eric; Coe, Fredric L. (13 January 2010)."Metabolic Acidosis after Sulfur Ingestion".New England Journal of Medicine.297(16): 869–870.doi:10.1056/nejm197710202971606.PMID904661.Retrieved2 September2022.
- ^"Sulfur Dioxide | Toxicological Profile | ATSDR".Centers for Disease Control and Prevention.26 March 2014.Retrieved24 October2023.
- ^"Sulfur General Fact Sheet".npic.orst.edu.Retrieved20 January2024.
- ^Baker, Colin (1 March 2007)."The dehydration of sucrose".Education in Chemistry.Royal Society of Chemistry.Retrieved14 June2018.
- ^"4.1: Fossil Fuels and Products of Combustion".Engineering LibreTexts.28 February 2021.Retrieved24 October2023.
- ^"Hydrogen Sulfide Toxicity: Practice Essentials, Pathophysiology, Etiology".Medscape.30 March 2017 – via eMedicine.
- ^Summers, Vincent (8 April 2017)."Hydrogen Sulfide or Hydrogen Cyanide: Which is More Dangerous?".Quirky Science.Retrieved23 August2022.
- ^"Hydrogen Sulfide – Hazards | Occupational Safety and Health Administration".www.osha.gov.Retrieved23 August2022.
Further reading
[edit]Sigel, Astrid; Freisinger, Eva; Sigel, Roland K.O., eds. (2020).Transition Metals and Sulfur: A Strong Relationship for Life.Guest Editors Martha E Sosa Torres and Peter M.H.Kroneck. Berlin/Boston: de Gruyter. pp. xlv+455.ISBN978-3-11-058889-7.
External links
[edit]- SulfuratThe Periodic Table of Videos(University of Nottingham)
- Atomic Data for Sulfur,NISTPhysical Measurement Laboratory
- Sulfur phase diagramArchived23 February 2010 at theWayback Machine,Introduction to Chemistry for Ages 13–17
- Crystalline, liquid and polymerization of sulfur on Vulcano Island, Italy
- Sulfur and its use as a pesticide
- The Sulphur Institute
- Nutrient Stewardship and The Sulphur Institute
- Sulfur
- Chemical elements
- Chalcogens
- Reactive nonmetals
- Polyatomic nonmetals
- Agricultural chemicals
- Anti-acne preparations
- Dietary minerals
- Industrial minerals
- Inorganic polymers
- Native element minerals
- Orthorhombic minerals
- Minerals in space group 70
- Pyrotechnic fuels
- Chemical elements with primitive orthorhombic structure