Surface energy

Insurface science,surface energy(alsointerfacial free energyorsurface free energy) quantifies the disruption ofintermolecular bondsthat occurs when asurfaceis created. Insolid-state physics,surfaces must be intrinsically lessenergetically favorablethan the bulk of the material (that is, the atoms on the surface must have more energy than the atoms in the bulk), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material bysublimation.The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is theworkrequired to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding between the two created surfaces.
Cutting a solid body into pieces disrupts its bonds and increases the surface area, and therefore increases surface energy. If the cutting is donereversibly,thenconservation of energymeans that the energy consumed by the cutting process will be equal to the energy inherent in the two new surfaces created. The unit surface energy of a material would therefore be half of its energy ofcohesion,all other things being equal; in practice, this is true only for a surface freshly prepared invacuum.Surfaces often change their form away from the simple "cleaved bond"model just implied above. They are found to be highly dynamic regions, which readily rearrange orreact,so that energy is often reduced by such processes aspassivationoradsorption.
Assessment
[edit]Measurement
[edit]Contact angle
[edit]The most common way to measure surface energy is throughcontact angleexperiments.[1]In this method, the contact angle of the surface is measured with several liquids, usually water anddiiodomethane.Based on the contact angle results and knowing thesurface tensionof the liquids, the surface energy can be calculated. In practice, this analysis is done automatically by a contact angle meter.[2]
There are several different models for calculating the surface energy based on the contact angle readings.[3]The most commonly used method is OWRK, which requires the use of two probe liquids and gives out as a result the total surface energy as well as divides it into polar and dispersive components.
Contact angle method is the standard surface energy measurement method due to its simplicity, applicability to a wide range of surfaces and quickness. The measurement can be fully automated and is standardized.[4]
In general, as surface energy increases, the contact angle decreases because more of the liquid is being "grabbed" by the surface. Conversely, as surface energy decreases, the contact angle increases, because the surface doesn't want to interact with the liquid.
Other methods
[edit]The surface energy of a liquid may be measured by stretching a liquidmembrane(which increases the surface area and hence the surface energy). In that case, in order to increase the surface area of a mass of liquid by an amount,δA,a quantity ofwork,γ δA,is needed (whereγis the surface energy density of the liquid). However, such a method cannot be used to measure the surface energy of a solid because stretching of a solid membrane induces elastic energy in the bulk in addition to increasing the surface energy.
The surface energy of a solid is usually measured at high temperatures. At such temperatures the solidcreepsand even though the surface area changes, the volume remains approximately constant. Ifγis the surface energy density of a cylindrical rod of radiusrand lengthlat high temperature and a constant uniaxial tensionP,then at equilibrium, thevariationof the totalHelmholtz free energyvanishes and we have
whereFis theHelmholtz free energyandAis the surface area of the rod:
Also, since the volume (V) of the rod remains constant, the variation (δV) of the volume is zero, that is,
Therefore, the surface energy density can be expressed as
The surface energy density of the solid can be computed by measuringP,r,andlat equilibrium.
This method is valid only if the solid isisotropic,meaning the surface energy is the same for allcrystallographicorientations. While this is only strictly true foramorphous solids(glass) and liquids, isotropy is a good approximation for many other materials. In particular, if the sample is polygranular (most metals) or made by powdersintering(most ceramics) this is a good approximation.
In the case of single-crystal materials, such as naturalgemstones,anisotropyin the surface energy leads tofaceting.The shape of the crystal (assumingequilibriumgrowth conditions) is related to the surface energy by theWulff construction.The surface energy of the facets can thus be found to within a scaling constant by measuring the relative sizes of the facets.
Calculation
[edit]Deformed solid
[edit]In the deformation of solids, surface energy can be treated as the "energy required to create one unit of surface area", and is a function of the difference between the total energies of the system before and after the deformation:
- .
Calculation of surface energyfrom first principles(for example,density functional theory) is an alternative approach to measurement. Surface energy is estimated from the following variables: width of the d-band, thenumber of valence d-electrons,and thecoordination numberof atoms at the surface and in the bulk of the solid.[5][page needed]
Surface formation energy of a crystalline solid
[edit]Indensity functional theory,surface energy can be calculated from the following expression:
where
- Eslabis the total energy of surface slab obtained using density functional theory.
- Nis the number of atoms in the surface slab.
- Ebulkis the bulk energy per atom.
- Ais the surface area.
For a slab, we have two surfaces and they are of the same type, which is reflected by the number 2 in the denominator. To guarantee this, we need to create the slab carefully to make sure that the upper and lower surfaces are of the same type.
Strength of adhesive contacts is determined by the work of adhesion which is also calledrelative surface energyof two contacting bodies.[6][page needed]The relative surface energy can be determined by detaching of bodies of well defined shape made of one material from the substrate made from the second material.[7]For example, the relative surface energy of the interface "acrylic glass–gelatin"is equal to 0.03 N/m. Experimental setup for measuring relative surface energy and its function can be seen in the video.[8]
Estimation from the heat of sublimation
[edit]To estimate the surface energy of a pure, uniform material, an individual region of the material can be modeled as a cube. In order to move a cube from the bulk of a material to the surface, energy is required. This energy cost is incorporated into the surface energy of the material, which is quantified by:

wherezσandzβare coordination numbers corresponding to the surface and the bulk regions of the material, and are equal to 5 and 6, respectively;a0is the surface area of an individual molecule, andWAAis the pairwise intermolecular energy.
Surface area can be determined by squaring the cube root of the volume of the molecule:
Here,M̄corresponds to themolar massof the molecule,ρcorresponds to the density, andNAis theAvogadro constant.
In order to determine the pairwise intermolecular energy, all intermolecular forces in the material must be broken. This allows thorough investigation of the interactions that occur for single molecules. During sublimation of a substance, intermolecular forces between molecules are broken, resulting in a change in the material from solid to gas. For this reason, considering theenthalpy of sublimationcan be useful in determining the pairwise intermolecular energy. Enthalpy of sublimation can be calculated by the following equation:
Using empirically tabulated values for enthalpy of sublimation, it is possible to determine the pairwise intermolecular energy. Incorporating this value into the surface energy equation allows for the surface energy to be estimated.
The following equation can be used as a reasonable estimate for surface energy:
Interfacial energy
[edit]The presence of aninterfaceinfluences generally all thermodynamic parameters of a system. There are two models that are commonly used to demonstrate interfacial phenomena: theGibbs ideal interfacemodel and the Guggenheim model. In order to demonstrate the thermodynamics of an interfacial system using the Gibbs model, the system can be divided into three parts: twoimmiscibleliquids with volumesVαandVβand an infinitesimally thin boundary layer known as the Gibbs dividing plane (σ) separating these two volumes.

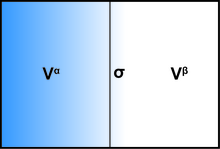
The total volume of the system is:
All extensive quantities of the system can be written as a sum of three components: bulk phaseα,bulk phaseβ,and the interfaceσ.Some examples include internal energyU,the number of molecules of theith substanceni,and the entropyS.
While these quantities can vary between each component, the sum within the system remains constant. At the interface, these values may deviate from those present within the bulk phases. Theconcentrationof molecules present at the interface can be defined as:
whereciαandciβrepresent the concentration of substanceiin bulk phaseαandβ,respectively.
It is beneficial to define a new term interfacial excessΓiwhich allows us to describe the number of molecules per unit area:
Wetting
[edit]Spreading parameter
[edit]Surface energy comes into play in wetting phenomena. To examine this, consider a drop of liquid on a solid substrate. If the surface energy of the substrate changes upon the addition of the drop, the substrate is said to bewetting.The spreading parameter can be used to mathematically determine this:
whereSis the spreading parameter,γsthe surface energy of the substrate,γlthe surface energy of the liquid, andγs-lthe interfacial energy between the substrate and the liquid.
IfS< 0,the liquid partially wets the substrate. IfS> 0,the liquid completely wets the substrate.[9]
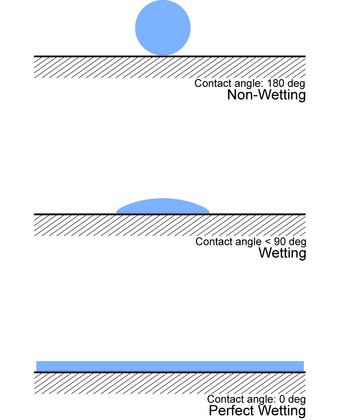
Contact angle
[edit]A way to experimentally determine wetting is to look at thecontact angle(θ), which is the angle connecting the solid–liquid interface and the liquid–gas interface (as in the figure).
- Ifθ= 0°,the liquid completely wets the substrate.
- If0° <θ< 90°,high wetting occurs.
- If90° <θ< 180°,low wetting occurs.
- Ifθ= 180°,the liquid does not wet the substrate at all.[10]
TheYoung equationrelates the contact angle to interfacial energy:
whereγs-gis the interfacial energy between the solid and gas phases,γs-lthe interfacial energy between the substrate and the liquid,γl-gis the interfacial energy between the liquid and gas phases, andθis the contact angle between the solid–liquid and the liquid–gas interface.[11]
Wetting of high- and low-energy substrates
[edit]The energy of the bulk component of a solid substrate is determined by the types of interactions that hold the substrate together. High-energy substrates are held together bybonds,while low-energy substrates are held together byforces.Covalent,ionic,andmetallic bondsare much stronger than forces such asvan der Waalsandhydrogen bonding.High-energy substrates are more easily wetted than low-energy substrates.[12]In addition, more complete wetting will occur if the substrate has a much higher surface energy than the liquid.[13]
Modification techniques
[edit]The most commonly used surface modification protocols areplasma activation,wet chemical treatment, including grafting, and thin-film coating.[14][15][16]Surface energy mimicking is a technique that enables merging the device manufacturing and surface modifications, including patterning, into a single processing step using a single device material.[17]
Many techniques can be used to enhance wetting. Surface treatments, such ascorona treatment,[18]plasma treatment andacid etching,[19]can be used to increase the surface energy of the substrate. Additives can also be added to the liquid to decrease its surface tension. This technique is employed often inpaintformulations to ensure that they will be evenly spread on a surface.[20]
The Kelvin equation
[edit]As a result of the surface tension inherent to liquids, curved surfaces are formed in order to minimize the area. This phenomenon arises from the energetic cost of forming a surface. As such the Gibbs free energy of the system is minimized when the surface is curved.

TheKelvin equationis based on thermodynamic principles and is used to describe changes in vapor pressure caused by liquids with curved surfaces. The cause for this change in vapor pressure is the Laplace pressure. The vapor pressure of a drop is higher than that of a planar surface because the increased Laplace pressure causes the molecules to evaporate more easily. Conversely, in liquids surrounding a bubble, the pressure with respect to the inner part of the bubble is reduced, thus making it more difficult for molecules to evaporate. The Kelvin equation can be stated as:
wherePK
0is thevapor pressureof the curved surface,P0is the vapor pressure of the flat surface,γis thesurface tension,Vmis themolar volumeof the liquid,Ris theuniversal gas constant,Tistemperature(inkelvin), andR1andR2are the principalradii of curvatureof the surface.
Surface modified pigments for coatings
[edit]Pigmentsoffer great potential in modifying the application properties of a coating. Due to their fine particle size and inherently high surface energy, they often require a surface treatment in order to enhance their ease of dispersion in a liquid medium. A wide variety of surface treatments have been previously used, including theadsorptionon the surface of a molecule in the presence of polar groups, monolayers of polymers, and layers of inorganic oxides on the surface of organic pigments.[21]
New surfaces are constantly being created as larger pigment particles get broken down into smaller subparticles. These newly-formed surfaces consequently contribute to larger surface energies, whereby the resulting particles often become cemented together into aggregates. Because particles dispersed in liquid media are in constant thermal orBrownian motion,they exhibit a strong affinity for other pigment particles nearby as they move through the medium and collide.[21]This natural attraction is largely attributed to the powerful short-rangevan der Waals forces,as an effect of their surface energies.
The chief purpose of pigment dispersion is to break down aggregates and form stable dispersions of optimally sized pigment particles. This process generally involves three distinct stages: wetting, deaggregation, and stabilization. A surface that is easy to wet is desirable when formulating a coating that requires good adhesion and appearance. This also minimizes the risks of surface tension related defects, such as crawling, cratering, andorange peel.[22]This is an essential requirement for pigment dispersions; for wetting to be effective, the surface tension of the pigment's vehicle must be lower than the surface free energy of the pigment.[21]This allows the vehicle to penetrate into the interstices of the pigment aggregates, thus ensuring complete wetting. Finally, the particles are subjected to a repulsive force in order to keep them separated from one another and lowers the likelihood offlocculation.
Dispersions may become stable through two different phenomena: charge repulsion and steric or entropic repulsion.[22]In charge repulsion, particles that possess the same like electrostatic charges repel each other. Alternatively,stericorentropic repulsionis a phenomenon used to describe the repelling effect when adsorbed layers of material (such as polymer molecules swollen with solvent) are present on the surface of the pigment particles in dispersion. Only certain portions (anchors) of the polymer molecules are adsorbed, with their corresponding loops and tails extending out into the solution. As the particles approach each other their adsorbed layers become crowded; this provides an effective steric barrier that preventsflocculation.[23]This crowding effect is accompanied by a decrease in entropy, whereby the number of conformations possible for the polymer molecules is reduced in the adsorbed layer. As a result, energy is increased and often gives rise to repulsive forces that aid in keeping the particles separated from each other.

Surface energies of common materials
[edit]| Material | Orientation | Surface energy (mJ/m2) |
|---|---|---|
| Polytetrafluoroethylene(PTFE) | 19[24][page needed] | |
| Glass | 83.4[25] | |
| Gypsum | 370[26] | |
| Copper | 1650[27] | |
| Magnesium oxide | (100) plane | 1200[28] |
| Calcium fluoride | (111) plane | 450[28] |
| Lithium fluoride | (100) plane | 340[28] |
| Calcium carbonate | (1010) plane | 230[28] |
| Sodium chloride | (100) plane | 300[29] |
| Sodium chloride | (110) plane | 400[30] |
| Potassium chloride | (100) plane | 110[29] |
| Barium fluoride | (111) plane | 280[28] |
| Silicon | (111) plane | 1240[28] |
See also
[edit]References
[edit]- ^Marshall, S. J.; Bayne, S. C.; Baier, R.; Tomsia, A. P.; Marshall, G. W. (2010). "A review of adhesion science".Dental Materials.26(2): e11–e16.doi:10.1016/j.dental.2009.11.157.PMID20018362.
- ^Laurén, S."How To Measure Surface Free Energy?".blog.biolinscientific.com.Biolin Scientific.Retrieved2019-12-31.
- ^"Surface Free Energy: Measurements".biolinscientific.com.Biolin Scientific.Retrieved2019-12-31.
- ^"ISO 19403-2:2017. Paints and varnishes — Wettability — Part 2: Determination of the surface free energy of solid surfaces by measuring the contact angle".ISO.2017.
- ^Woodruff, D. P., ed. (2002).The Chemical Physics of Solid Surfaces.Vol. 10. Elsevier.[ISBN missing]
- ^Contact Mechanics and Friction: Physical Principles and Applications.Springer. 2017.ISBN9783662530801.
- ^Popov, V. L.; Pohrt, R.; Li, Q. (September 2017)."Strength of adhesive contacts: Influence of contact geometry and material gradients".Friction.5(3): 308–325.doi:10.1007/s40544-017-0177-3.
- ^Dept. of System Dynamics and Friction Physics (December 6, 2017)."Science friction: Adhesion of complex shapes".YouTube.Archivedfrom the original on 2021-12-12.Retrieved2018-01-28.
- ^Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. (2009)."Wetting and Spreading".Reviews of Modern Physics.81(2): 739–805.Bibcode:2009RvMP...81..739B.doi:10.1103/revmodphys.81.739.
- ^Zisman, W. (1964). "Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution".Contact Angle, Wettability, and Adhesion.Advances in Chemistry. Vol. 43. pp. 1–51.doi:10.1021/ba-1964-0043.ch001.ISBN0-8412-0044-0.
- ^Owens, D. K.; Wendt, R. C. (1969). "Estimation of the Surface Free Energy of Polymers".Journal of Applied Polymer Science.13(8): 1741–1747.doi:10.1002/app.1969.070130815.
- ^De Gennes, P. G. (1985). "Wetting: statics and dynamics".Reviews of Modern Physics.57(3): 827–863.Bibcode:1985RvMP...57..827D.doi:10.1103/revmodphys.57.827.
- ^Kern, K.; David, R.; Palmer, R. L.; Cosma, G. (1986). "Complete Wetting on 'Strong' Substrates: Xe/Pt(111)".Physical Review Letters.56(26): 2823–2826.Bibcode:1986PhRvL..56.2823K.doi:10.1103/physrevlett.56.2823.PMID10033104.
- ^ Becker, H.; Gärtner, C. (2007). "Polymer microfabrication technologies for microfluidic systems".Analytical and Bioanalytical Chemistry.390(1): 89–111.doi:10.1007/s00216-007-1692-2.PMID17989961.S2CID13813183.
- ^ Mansky (1997). "Controlling Polymer-Surface Interactions with Random Copolymer Brushes".Science.275(5305): 1458–1460.doi:10.1126/science.275.5305.1458.S2CID136525970.
- ^ Rastogi (2010). "Direct Patterning of Intrinsically Electron Beam Sensitive Polymer Brushes".ACS Nano.4(2): 771–780.doi:10.1021/nn901344u.PMID20121228.
- ^ Pardon, G.; Haraldsson, T.; van der Wijngaart, W. (2016)."Surface Energy Mimicking: Simultaneous Replication of Hydrophilic and Superhydrophobic Micropatterns through Area-Selective Monomers Self-Assembly".Advanced Materials Interfaces.3(17): 1600404.doi:10.1002/admi.201600404.S2CID138114323.
- ^Sakata, I.; Morita, M.; Tsuruta, N.; Morita, K. (2003). "Activation of Wood Surface by Corona Treatment to Improve Adhesive Bonding".Journal of Applied Polymer Science.49(7): 1251–1258.doi:10.1002/app.1993.070490714.
- ^Rosales, J. I.; Marshall, G. W.; Marshall, S. J.; Wantanabe, L. G.; Toledano, M.; Cabrerizo, M. A.; Osorio, R. (1999). "Acid-etching and Hydration Influence on Dentin Roughness and Wettability".Journal of Dental Research.78(9): 1554–1559.doi:10.1177/00220345990780091001.PMID10512390.S2CID5807073.
- ^Khan, H.; Fell, J. T.; Macleod, G. S. (2001). "The influence of additives on the spreading coefficient and adhesion of a film coating formulation to a model tablet surface".International Journal of Pharmaceutics.227(1–2): 113–119.doi:10.1016/s0378-5173(01)00789-x.PMID11564545.
- ^abcWicks, Z. W. (2007).Organic Coatings: Science and Technology(3rd ed.). New York: Wiley Interscience. pp. 435–441.[ISBN missing]
- ^abTracton, A. A. (2006).Coatings Materials and Surface Coatings(3rd ed.). Florida: Taylor and Francis Group. pp. 31-6–31-7.[ISBN missing]
- ^Auschra, C.; Eckstein, E.; Muhlebach, A.; Zink, M.; Rime, F. (2002). "Design of new pigment dispersants by controlled radical polymerization".Progress in Organic Coatings.45(2–3): 83–93.doi:10.1016/s0300-9440(02)00048-6.
- ^Kinloch, A. J. (1987).Adhesion & Adhesives: Science & Technology.London: Chapman & Hall.[ISBN missing]
- ^Rhee, S.-K. (1977). "Surface energies of silicate glasses calculated from their wettability data".Journal of Materials Science.12(4): 823–824.Bibcode:1977JMatS..12..823R.doi:10.1007/BF00548176.S2CID136812418.
- ^Dundon, M. L.; Mack, E. (1923). "The Solubility and Surface Energy of Calcium Sulfate".Journal of the American Chemical Society.45(11): 2479–2485.doi:10.1021/ja01664a001.
- ^Udin, H. (1951)."Grain Boundary Effect in Surface Tension Measurement".JOM.3(1): 63.Bibcode:1951JOM.....3a..63U.doi:10.1007/BF03398958.
- ^abcdefGilman, J. J. (1960). "Direct Measurements of the Surface Energies of Crystals".Journal of Applied Physics.31(12): 2208.Bibcode:1960JAP....31.2208G.doi:10.1063/1.1735524.
- ^abButt, H.-J.; Graf, Kh.; Kappl, M. (2006).Physics and Chemistry of Interfaces.Weinheim: Wiley-VCH.[ISBN missing]
- ^Lipsett, S. G.; Johnson, F. M. G.; Maass, O. (1927). "The Surface Energy and the Heat of Solution of Solid Sodium Chloride. I".Journal of the American Chemical Society.49(4): 925.doi:10.1021/ja01403a005.

















