Sex-determining region Y protein

Sex-determining region Y protein(SRY), ortestis-determining factor(TDF), is aDNA-binding protein(also known as gene-regulatory protein/transcription factor) encoded by theSRYgenethat is responsible for the initiation of malesex determinationintherianmammals(placental mammalsandmarsupials).[5]SRY is anintronlesssex-determining gene on theY chromosome.[6]Mutationsin this gene lead to a range ofdisorders of sex developmentwith varying effects on an individual'sphenotypeandgenotype.
SRY is a member of theSOX (SRY-like box) genefamily ofDNA-binding proteins. When complexed with the(SF-1) protein,SRY acts as a transcription factor that causesupregulationof other transcription factors, most importantlySOX9.[7]Itsexpressioncauses the development of primarysex cords,which later develop intoseminiferous tubules.These cords form in the central part of the yet-undifferentiatedgonad,turning it into atestis.The now-inducedLeydig cellsof the testis then start secretingtestosterone,while theSertoli cellsproduceanti-Müllerian hormone.[8]SRY gene effects normally take place 6–8 weeks after fetus formation which inhibits the female anatomical structural growth in males. It also works towards developing thesecondary sexual characteristicsof males.
Gene evolution and regulation[edit]
Evolution[edit]
SRYmay have arisen from agene duplicationof theX chromosomebound geneSOX3,a member of theSOX family.[9][10]This duplication occurred after the split betweenmonotremesandtherians.Monotremes lack SRY and some of their sex chromosomes share homology with bird sex chromosomes.[11]SRYis a quickly evolving gene, and its regulation has been difficult to study because sex determination is not a highly conserved phenomenon within the animal kingdom.[12]Even withinmarsupialsandplacentals,which useSRYin their sex determination process, the action ofSRYdiffers between species.[10]The gene sequence also changes; while the core of the gene, thehigh-mobility group(HMG) box,is conserved between species, other regions of the gene are not.[10]SRYis one of only four genes on the human Y chromosome that have been shown to have arisen from the original Y chromosome.[13]The other genes on the human Y chromosome arose from anautosomethat fused with the original Y chromosome.[13]
Regulation[edit]
SRYhas little in common with sex determination genes of other model organisms, therefore, mice are the main model research organisms that can be utilized for its study. Understanding its regulation is further complicated because even between mammalian species, there is little proteinsequence conservation.The only conserved group in mice and other mammals is the HMG box region that is responsible for DNA binding. Mutations in this region result insex reversal,where the opposite sex is produced.[14]Because there is little conservation, theSRYpromoter,regulatory elements and regulation are not well understood. Within related mammalian groups there are homologies within the first 400–600base pairs(bp) upstream from thetranslational start site.In vitro studies of humanSRYpromoter have shown that a region of at least 310 bp upstream to translational start site are required forSRYpromoter function. It has been shown that binding of three transcription factors, steroidogenic factor 1 (SF1), specificity protein 1 (Sp1 transcription factor) and Wilms tumor protein 1 (WT1), to the human promoter sequence, influence expression ofSRY.[14]
The promoter region has two Sp1 binding sites, at -150 and -13 that function as regulatory sites. Sp1 is a transcription factor that binds GC-rich consensus sequences, and mutation of theSRYbinding sites leads to a 90% reduction in gene transcription. Studies of SF1 have resulted in less definite results. Mutations of SF1 can lead to sex reversal, and deletion can lead to incomplete gonad development. However, it is not clear how SF1 interacts with theSR1promoter directly.[15]The promoter region also has two WT1 binding sites at -78 and -87 bp from the ATG codon. WT1 is transcription factor that has four C-terminalzinc fingersand an N-terminal Pro/Glu-rich region and primarily functions as an activator. Mutation of the zinc fingers or inactivation of WT1 results in reduced male gonad size. Deletion of the gene resulted in complete sex reversal. It is not clear how WT1 functions to up-regulateSRY,but some research suggests that it helps stabilize message processing.[15]However, there are complications to this hypothesis, because WT1 also is responsible for expression of an antagonist of male development,DAX1,which stands for dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1. An additional copy of DAX1 in mice leads to sex reversal. It is not clear how DAX1 functions, and many different pathways have been suggested, includingSRYtranscriptional destabilization and RNA binding. There is evidence from work on suppression of male development that DAX1 can interfere with function of SF1, and in turn transcription ofSRYby recruiting corepressors.[14]
There is also evidence that GATA binding protein 4 (GATA4) and FOG2 contribute to activation ofSRYby associating with its promoter. How these proteins regulateSRYtranscription is not clear, but FOG2 and GATA4 mutants have significantly lower levels ofSRYtranscription.[16]FOGs have zinc finger motifs that can bind DNA, but there is no evidence of FOG2 interaction withSRY.Studies suggest that FOG2 and GATA4 associate with nucleosome remodeling proteins that could lead to its activation.[17]
Function[edit]
During gestation, the cells of the primordial gonad that lie along theurogenital ridgeare in a bipotential state, meaning they possess the ability to become either male cells (SertoliandLeydigcells) or female cells (folliclecells andthecacells). SRY initiates testis differentiation by activating male-specific transcription factors that allow these bipotential cells to differentiate and proliferate. SRY accomplishes this by upregulatingSOX9,a transcription factor with a DNA-binding site very similar to SRY's. SOX9 leads to the upregulation of fibroblast growth factor 9 (Fgf9), which in turn leads to further upregulation of SOX9. Once proper SOX9 levels are reached, the bipotential cells of the gonad begin to differentiate into Sertoli cells. Additionally, cells expressing SRY will continue to proliferate to form the primordial testis. This brief review constitutes the basic series of events, but there are many more factors that influence sex differentiation.
Action in the nucleus[edit]
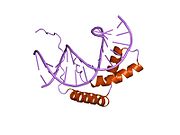
The SRY protein consists of three main regions. The central region encompasses thehigh-mobility group(HMG) domain, which containsnuclear localization sequencesand acts as the DNA-binding domain. TheC-terminaldomain has no conserved structure, and theN-terminaldomain can be phosphorylated to enhance DNA-binding.[15]The process begins withnuclear localizationof SRY byacetylationof the nuclear localization signal regions, which allows for the binding ofimportin βandcalmodulinto SRY, facilitating its import into the nucleus. Once in the nucleus, SRY and SF1 (steroidogenic factor 1,another transcriptional regulator) complex and bind to TESCO (testis-specific enhancer of Sox9 core), the testes-specific enhancer element of the Sox9 gene in Sertoli cell precursors, located upstream of the Sox9 gene transcription start site.[7]Specifically, it is the HMG region of SRY that binds to the minor groove of the DNA target sequence, causing the DNA to bend and unwind. The establishment of this particular DNA "architecture" facilitates the transcription of the Sox9 gene.[15]In the nucleus of Sertoli cells, SOX9 directly targets theAmhgene as well as theprostaglandin D synthase(Ptgds)gene. SOX9 binding to the enhancer near theAmhpromoter allows for the synthesis ofAmhwhile SOX9 binding to thePtgdsgene allows for the production ofprostaglandin D2(PGD2). The reentry of SOX9 into the nucleus is facilitated by autocrine or paracrine signaling conducted by PGD2.[18]SOX9 protein then initiates apositive feedbackloop, involving SOX9 acting as its own transcription factor and resulting in the synthesis of large amounts of SOX9.[15]
SOX9 and testes differentiation[edit]
The SF-1 protein, on its own, leads to minimal transcription of the SOX9 gene in both the XX and XY bipotential gonadal cells along the urogenital ridge. However, binding of the SRY-SF1 complex to the testis-specific enhancer (TESCO) on SOX9 leads to significant up-regulation of the gene in only the XY gonad, while transcription in the XX gonad remains negligible. Part of this up-regulation is accomplished by SOX9 itself through a positive feedback loop; like SRY, SOX9 complexes with SF1 and binds to the TESCO enhancer, leading to further expression of SOX9 in the XY gonad. Two other proteins,FGF9(fibroblast growth factor 9) and PDG2 (prostaglandin D2), also maintain this up-regulation. Although their exact pathways are not fully understood, they have been proven to be essential for the continued expression of SOX9 at the levels necessary for testes development.[7]
SOX9 and SRY are believed to be responsible for the cell-autonomous differentiation of supporting cell precursors in the gonads into Sertoli cells, the beginning of testes development. These initial Sertoli cells, in the center of the gonad, are hypothesized to be the starting point for a wave of FGF9 that spreads throughout the developing XY gonad, leading to further differentiation of Sertoli cells via the up-regulation of SOX9.[19]SOX9 and SRY are also believed to be responsible for many of the later processes of testis development (such as Leydig cell differentiation, sex cord formation, and formation of testis-specific vasculature), although exact mechanisms remain unclear.[20]It has been shown, however, that SOX9, in the presence of PDG2, acts directly on Amh (encoding anti-Müllerian hormone) and is capable of inducing testis formation in XX mice gonads, indicating it is vital to testes development.[19]
SRY disorders' influence on sex expression[edit]
Embryos are gonadally identical, regardless of genetic sex, until a certain point in development when the testis-determining factor causes male sex organs to develop. A typical malekaryotypeis XY, whereas a female's is XX. There are exceptions, however, in which SRY plays a major role. Individuals withKlinefelter syndromeinherit a normal Y chromosome and multiple X chromosomes, giving them a karyotype of XXY. Atypicalgenetic recombinationduringcrossover,when a sperm cell is developing, can result in karyotypes that are not typical for their phenotypic expression.
Most of the time, when a developing sperm cell undergoes crossover during meiosis, the SRY gene stays on the Y chromosome. If the SRY gene is transferred to the X chromosome instead of staying on the Y chromosome, testis development will no longer occur. This is known asSwyer syndrome,characterized by an XY karyotype and a female phenotype. Individuals who have this syndrome have normally formed uteri and fallopian tubes, but the gonads are not functional. Swyer syndrome individuals are usually considered as females.[21]On the other spectrum,XX male syndromeoccurs when a body has 46:XX Karyotype and SRY attaches to one of them through translocation. People with XX male syndrome have a XX Karyotype but are male.[22]Individuals with either of these syndromes can experience delayed puberty, infertility, and growth features of the opposite sex they identify with. XX male syndrome expressers may develop breasts, and those with Swyer syndrome may have facial hair.[21][23]
| Klinefelter Syndrome |
|
| Swyer Syndrome |
|
| XX Male Syndrome |
|
While the presence or absence of SRY has generally determined whether or not testis development occurs, it has been suggested that there are other factors that affect the functionality of SRY.[24]Therefore, there are individuals who have the SRY gene, but still develop as females, either because the gene itself is defective or mutated, or because one of the contributing factors is defective.[25]This can happen in individuals exhibiting a XY, XXY, or XX SRY-positive karyotype.
Additionally, other sex determining systems that rely on SRY beyond XY are the processes that come after SRY is present or absent in the development of an embryo. In a normal system, if SRY is present for XY, SRY will activate the medulla to develop gonads into testes. Testosterone will then be produced and initiate the development of other male sexual characteristics. Comparably, if SRY is not present for XX, there will be a lack of the SRY based on no Y chromosome. The lack of SRY will allow the cortex of embryonic gonads to develop into ovaries, which will then produce estrogen, and lead to the development of other female sexual characteristics.[26]
Role in other diseases[edit]
SRY has been shown tointeractwith theandrogen receptorand individuals with XY karyotype and a functional SRY gene can have an outwardly female phenotype due to an underlyingandrogen insensitivity syndrome(AIS).[27]Individuals with AIS are unable to respond to androgens properly due to a defect in their androgen receptor gene, and affected individuals can have complete or partial AIS.[28]SRY has also been linked to the fact that males are more likely than females to developdopamine-related diseases such asschizophreniaandParkinson's disease.SRY encodes a protein that controls the concentration of dopamine, the neurotransmitter that carries signals from the brain that control movement and coordination.[29]Research in mice has shown that a mutation in SOX10, an SRY encoded transcription factor, is linked to the condition of Dominant megacolon in mice.[30]This mouse model is being used to investigate the link between SRY andHirschsprung disease,or congenital megacolon in humans.[30]There is also a link between SRY encoded transcription factor SOX9 andcampomelic dysplasia(CD).[31]This missense mutation causes defectivechondrogenesis,or the process of cartilage formation, and manifests as skeletal CD.[32]Two thirds of 46,XY individuals diagnosed with CD have fluctuating amounts of male-to-female sex reversal.[31]
Use in Olympic screening[edit]
One of the most controversial uses of this discovery was as a means forgender verificationat theOlympic Games,under a system implemented by theInternational Olympic Committeein 1992. Athletes with an SRY gene were not permitted to participate as females, although all athletes in whom this was "detected" at the1996 Summer Olympicswere ruledfalse positivesand were not disqualified. Specifically, eight female participants (out of a total of 3387) at these games were found to have the SRY gene. However, after further investigation of their genetic conditions, all these athletes were verified as female and allowed to compete. These athletes were found to have either partial or fullandrogen insensitivity,despite having an SRY gene, making them externally phenotypically female.[33]In the late 1990s, a number of relevant professional societies in United States called for elimination of gender verification, including theAmerican Medical Association,stating that the method used was uncertain and ineffective.[34]Chromosomal screening was eliminated as of the2000 Summer Olympics,[34][35][36]but this was later followed by other forms of testing based on hormone levels.[37]
Ongoing research[edit]
Despite the progress made during the past several decades in the study of sex determination, the SRY gene, and its protein, work is still being conducted to further understanding in these areas. There remain factors that need to be identified in the sex-determining molecular network, and the chromosomal changes involved in many other human sex-reversal cases are still unknown. Scientists continue to search for additional sex-determining genes, using techniques such asmicroarrayscreening of the genital ridge genes at varying developmental stages, mutagenesis screens in mice for sex-reversal phenotypes, and identifying the genes that transcription factors act on usingchromatin immunoprecipitation.[15]
Fetal Development- Knockout Models[edit]
One of the knockout models for the SRY gene was done in pigs. Through the use of CRISPR technology the SRY gene was knocked out in male pigs. The target for the CRISPR technology is the high mobility group located on the SRY gene. The research showed that with the absence of SRY, both the internal and external genitalia were reversed. When the piglets were born they were phenotypically male but expressed female genitalia.[38]Another study done on mice used TALEN technology to produce an SRY knockout model. These mice expressed external and internal genitalia as well as a normal female level of circulating testosterone.[39]These mice, despite having XY chromosomes, expressed a normal estrus cycle albeit with reduced fertility. Both of these studies highlighted the role that SRY plays in the development of the testes and other male reproductive organs.
See also[edit]
References[edit]
- ^abcGRCh38: Ensembl release 89: ENSG00000184895–Ensembl,May 2017
- ^abcGRCm38: Ensembl release 89: ENSMUSG00000069036–Ensembl,May 2017
- ^"Human PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Mouse PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, et al. (November 1990). "Genetic evidence equating SRY and the testis-determining factor".Nature.348(6300): 448–50.Bibcode:1990Natur.348..448B.doi:10.1038/348448A0.PMID2247149.S2CID3336314.
- ^Wallis MC, Waters PD, Graves JA (October 2008)."Sex determination in mammals--before and after the evolution of SRY".Cellular and Molecular Life Sciences.65(20): 3182–95.doi:10.1007/s00018-008-8109-z.PMC11131626.PMID18581056.S2CID31675679.
- ^abcKashimada K, Koopman P (December 2010)."Sry: the master switch in mammalian sex determination".Development.137(23): 3921–30.doi:10.1242/dev.048983.PMID21062860.
- ^Mittwoch U (October 1988). "The race to be male".New Scientist.120(1635): 38–42.
- ^Katoh K, Miyata T (December 1999). "A heuristic approach of maximum likelihood method for inferring phylogenetic tree and an application to the mammalian SOX-3 origin of the testis-determining gene SRY".FEBS Letters.463(1–2): 129–32.doi:10.1016/S0014-5793(99)01621-X.PMID10601652.S2CID24519808.
- ^abcBakloushinskaya, I Y (2009). "Evolution of sex determination in mammals".Biology Bulletin.36(2): 167–174.Bibcode:2009BioBu..36..167B.doi:10.1134/S1062359009020095.S2CID36988324.
- ^Veyrunes F, Waters PD, Miethke P, Rens W, McMillan D, Alsop AE, et al. (June 2008)."Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes".Genome Research.18(6): 965–73.doi:10.1101/gr.7101908.PMC2413164.PMID18463302.
- ^Bowles J, Schepers G, Koopman P (November 2000)."Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators".Developmental Biology.227(2): 239–55.doi:10.1006/dbio.2000.9883.PMID11071752.
- ^abGraves JA (December 2015). "Weird mammals provide insights into the evolution of mammalian sex chromosomes and dosage compensation".Journal of Genetics.94(4): 567–74.doi:10.1007/s12041-015-0572-3.PMID26690510.S2CID186238659.
- ^abcEly D, Underwood A, Dunphy G, Boehme S, Turner M, Milsted A (November 2010)."Review of the Y chromosome, Sry and hypertension".Steroids.75(11): 747–53.doi:10.1016/j.steroids.2009.10.015.PMC2891862.PMID19914267.
- ^abcdefHarley VR, Clarkson MJ, Argentaro A (August 2003)."The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9]".Endocrine Reviews.24(4): 466–87.doi:10.1210/er.2002-0025.PMID12920151.
- ^Knower KC, Kelly S, Harley VR (2003). "Turning on the male--SRY, SOX9 and sex determination in mammals".Cytogenetic and Genome Research.101(3–4): 185–98.doi:10.1159/000074336.PMID14684982.S2CID20940513.
- ^Zaytouni T, Efimenko EE, Tevosian SG (2011).GATA Transcription Factors in the Developing Reproductive System.Advances in Genetics. Vol. 76. pp. 93–134.doi:10.1016/B978-0-12-386481-9.00004-3.ISBN9780123864819.PMID22099693.
- ^Sekido R, Lovell-Badge R (January 2009). "Sex determination and SRY: down to a wink and a nudge?".Trends in Genetics.25(1): 19–29.doi:10.1016/j.tig.2008.10.008.PMID19027189.
- ^abMcClelland K, Bowles J, Koopman P (January 2012)."Male sex determination: insights into molecular mechanisms".Asian Journal of Andrology.14(1): 164–71.doi:10.1038/aja.2011.169.PMC3735148.PMID22179516.
- ^Sekido R, Lovell-Badge R (2013)."Genetic control of testis development".Sexual Development.7(1–3): 21–32.doi:10.1159/000342221.PMID22964823.
- ^ab"Swyer syndrome".Genetics Home Reference.National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services.Retrieved3 March2020.
- ^"XX Male Syndrome {".encyclopedia.com.Retrieved3 March2020.
- ^"46,XX testicular disorder of sex development".Genetics Home Reference.National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services.Retrieved3 March2020.
- ^Polanco JC, Koopman P (February 2007). "Sry and the hesitant beginnings of male development".Developmental Biology.302(1): 13–24.doi:10.1016/j.ydbio.2006.08.049.PMID16996051.
- ^Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ (May 2009)."Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene".American Journal of Human Genetics.84(5): 658–63.doi:10.1016/j.ajhg.2009.03.016.PMC2680992.PMID19361780.
- ^Marieb EN, Hoehn K (2018).Human Anatomy & Physiology(Eleventh ed.). Hoboken, New Jersey: Pearson Education Limited.ISBN978-0-13-458099-9.OCLC1004376412.
- ^Yuan X, Lu ML, Li T, Balk SP (December 2001)."SRY interacts with and negatively regulates androgen receptor transcriptional activity".The Journal of Biological Chemistry.276(49): 46647–54.doi:10.1074/jbc.M108404200.PMID11585838.
- ^Lister Hill National Center for Biomedical Communications (2008)."Androgen insensitivity syndrome".Genetics Home Reference.U.S. National Library of Medicine.
- ^Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, et al. (February 2006)."Direct regulation of adult brain function by the male-specific factor SRY".Current Biology.16(4): 415–20.Bibcode:2006CBio...16..415D.doi:10.1016/j.cub.2006.01.017.PMID16488877.S2CID5939578.
- ^abHerbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans-Borgmeyer I, Puliti A, et al. (1998)."Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease".Proceedings of the National Academy of Sciences.95(9): 5161–5165.Bibcode:1998PNAS...95.5161H.doi:10.1073/pnas.95.9.5161.PMC20231.PMID9560246.
- ^abPritchett J, Athwal V, Roberts N, Hanley NA, Hanley KP (2011). "Understanding the role of SOX9 in acquired diseases: lessons from development".Trends in Molecular Medicine.17(3): 166–174.doi:10.1016/j.molmed.2010.12.001.PMID21237710.
- ^"OMIM Entry – # 114290 – CAMPOMELIC DYSPLASIA".omim.org.Retrieved29 February2020.
- ^"Olympic Gender Testing".
- ^abFacius GM (1 August 2004)."The Major Medical Blunder of the 20th Century".Gender Testing.facius-homepage.dk. Archived fromthe originalon 26 January 2010.Retrieved12 June2011.[self-published source?]
- ^Elsas LJ, Ljungqvist A, Ferguson-Smith MA, Simpson JL, Genel M, Carlson AS, et al. (2000)."Gender verification of female athletes".Genetics in Medicine.2(4): 249–54.doi:10.1097/00125817-200007000-00008.PMID11252710.
- ^Dickinson BD, Genel M, Robinowitz CB, Turner PL, Woods GL (October 2002)."Gender verification of female Olympic athletes".Medicine and Science in Sports and Exercise.34(10): 1539–42, discussion 1543.doi:10.1097/00005768-200210000-00001.PMID12370551.
- ^"IOC Regulations on Female Hyperandrogenism"(PDF).International Olympic Committee. 22 June 2012.Archived(PDF)from the original on 13 August 2012.Retrieved9 August2012.
- ^Kurtz S, Lucas-Hahn A, Schlegelberger B, Göhring G, Niemann H, Mettenleiter TC, et al. (January 2021)."Knockout of the HMG domain of the porcine SRY gene causes sex reversal in gene-edited pigs".Proceedings of the National Academy of Sciences of the United States of America.118(2).Bibcode:2021PNAS..11808743K.doi:10.1073/pnas.2008743118.PMC7812820.PMID33443157.
- ^Kato T, Miyata K, Sonobe M, Yamashita S, Tamano M, Miura K, et al. (November 2013)."Production of Sry knockout mouse using TALEN via oocyte injection".Scientific Reports.3(1): 3136.Bibcode:2013NatSR...3E3136K.doi:10.1038/srep03136.PMC3817445.PMID24190364.
Further reading[edit]
- Haqq CM, King CY, Ukiyama E, Falsafi S, Haqq TN, Donahoe PK, et al. (December 1994). "Molecular basis of mammalian sexual determination: activation of Müllerian inhibiting substance gene expression by SRY".Science.266(5190): 1494–500.Bibcode:1994Sci...266.1494H.doi:10.1126/science.7985018.PMID7985018.
- Goodfellow PN, Lovell-Badge R (1993). "SRY and sex determination in mammals".Annual Review of Genetics.27(1): 71–92.doi:10.1146/annurev.ge.27.120193.000443.PMID8122913.
- Hawkins JR (1993)."Mutational analysis of SRY in XY females".Human Mutation.2(5): 347–50.doi:10.1002/humu.1380020504.PMID8257986.S2CID43503112.
- Harley VR (2002). "The Molecular Action of Testis-Determining Factors SRY and SOX9".The Genetics and Biology of Sex Determination.Novartis Foundation Symposia. Vol. 244. pp. 57–66, discussion 66–7, 79–85, 253–7.doi:10.1002/0470868732.ch6.ISBN978-0-470-86873-7.PMID11990798.
- Jordan BK, Vilain E (2002). "SRY and the Genetics of Sex Determination".Pediatric Gender Assignment.Advances in Experimental Medicine and Biology. Vol. 511. pp. 1–13, discussion 13–4.doi:10.1007/978-1-4615-0621-8_1.ISBN978-1-4613-5162-7.PMID12575752.
- Oh HJ, Lau YF (March 2006). "KRAB: a partner for SRY action on chromatin".Molecular and Cellular Endocrinology.247(1–2): 47–52.doi:10.1016/j.mce.2005.12.011.PMID16414182.S2CID19870331.
- Polanco JC, Koopman P (February 2007). "Sry and the hesitant beginnings of male development".Developmental Biology.302(1): 13–24.doi:10.1016/j.ydbio.2006.08.049.PMID16996051.
- Hawkins JR, Taylor A, Berta P, Levilliers J, Van der Auwera B, Goodfellow PN (February 1992). "Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal".Human Genetics.88(4): 471–4.doi:10.1007/BF00215684.PMID1339396.S2CID9332496.
- Hawkins JR, Taylor A, Goodfellow PN, Migeon CJ, Smith KD, Berkovitz GD (November 1992)."Evidence for increased prevalence of SRY mutations in XY females with complete rather than partial gonadal dysgenesis".American Journal of Human Genetics.51(5): 979–84.PMC1682856.PMID1415266.
- Ferrari S, Harley VR, Pontiggia A, Goodfellow PN, Lovell-Badge R, Bianchi ME (December 1992)."SRY, like HMG1, recognizes sharp angles in DNA".The EMBO Journal.11(12): 4497–506.doi:10.1002/j.1460-2075.1992.tb05551.x.PMC557025.PMID1425584.
- Jäger RJ, Harley VR, Pfeiffer RA, Goodfellow PN, Scherer G (December 1992). "A familial mutation in the testis-determining gene SRY shared by both sexes".Human Genetics.90(4): 350–5.doi:10.1007/BF00220457.PMID1483689.S2CID19470332.
- Vilain E, McElreavey K, Jaubert F, Raymond JP, Richaud F, Fellous M (May 1992)."Familial case with sequence variant in the testis-determining region associated with two sex phenotypes".American Journal of Human Genetics.50(5): 1008–11.PMC1682588.PMID1570829.
- Müller J, Schwartz M, Skakkebaek NE (July 1992). "Analysis of the sex-determining region of the Y chromosome (SRY) in sex reversed patients: point-mutation in SRY causing sex-reversion in a 46,XY female".The Journal of Clinical Endocrinology and Metabolism.75(1): 331–3.doi:10.1210/jcem.75.1.1619028.PMID1619028.
- McElreavey KD, Vilain E, Boucekkine C, Vidaud M, Jaubert F, Richaud F, et al. (July 1992). "XY sex reversal associated with a nonsense mutation in SRY".Genomics.13(3): 838–40.doi:10.1016/0888-7543(92)90164-N.PMID1639410.
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, et al. (July 1990). "A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif".Nature.346(6281): 240–4.Bibcode:1990Natur.346..240S.doi:10.1038/346240a0.PMID1695712.S2CID4364032.
- Berkovitz GD, Fechner PY, Zacur HW, Rock JA, Snyder HM, Migeon CJ, et al. (November 1991)."Clinical and pathologic spectrum of 46,XY gonadal dysgenesis: its relevance to the understanding of sex differentiation".Medicine.70(6): 375–83.doi:10.1097/00005792-199111000-00003.PMID1956279.S2CID37972412.
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, et al. (November 1990). "Genetic evidence equating SRY and the testis-determining factor".Nature.348(6300): 448–50.Bibcode:1990Natur.348..448B.doi:10.1038/348448A0.PMID2247149.S2CID3336314.
- Jäger RJ, Anvret M, Hall K, Scherer G (November 1990). "A human XY female with a frame shift mutation in the candidate testis-determining gene SRY".Nature.348(6300): 452–4.Bibcode:1990Natur.348..452J.doi:10.1038/348452a0.PMID2247151.S2CID4326539.
- Ellis NA, Goodfellow PJ, Pym B, Smith M, Palmer M, Frischauf AM, et al. (January 1989). "The pseudoautosomal boundary in man is defined by an Alu repeat sequence inserted on the Y chromosome".Nature.337(6202): 81–4.Bibcode:1989Natur.337...81E.doi:10.1038/337081a0.PMID2909893.S2CID2890077.
- Whitfield LS, Hawkins TL, Goodfellow PN, Sulston J (May 1995). "41 kilobases of analyzed sequence from the pseudoautosomal and sex-determining regions of the short arm of the human Y chromosome".Genomics.27(2): 306–11.doi:10.1006/geno.1995.1047.PMID7557997.
- Délot EC, Vilain EJ (1993)."Nonsyndromic 46,XX Testicular Disorders/Differences of Sex Development".In Adam MP, Feldman J, Mirzaa GM, et al. (eds.).GeneReviews.University of Washington, Seattle.PMID20301589.
External links[edit]
- Genes,+sryat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- Sex-Determining+Region+Y+Proteinat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- OMIM entries on 46,XX Testicular Disorder of Sex Development
- PDBe-KBprovides an overview of all the structure information available in the PDB for Human Sex-determining region Y protein