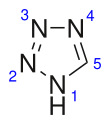
Tetrazole
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.477 | ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| CH2N4 | |||
| Molar mass | 70.05 g/mol | ||
| Density | 1.477 g/mL | ||
| Melting point | 157 to 158 °C (315 to 316 °F; 430 to 431 K)[2] | ||
| Boiling point | 220 ± 23 °C (428 ± 41 °F; 493 ± 23 K) | ||
| Acidity(pKa) | 4.90[1] | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Tetrazolesare a class ofsyntheticorganicheterocyclic compound,consisting of a 5-member ring of fournitrogenatoms and onecarbonatom. The name tetrazole also refers to the parent compound with formula CH2N4,of which three isomers can be formulated.
Structure and bonding
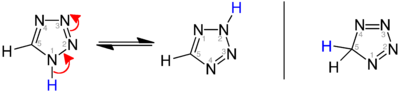
[edit]Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1H-, 2H-, and 5H-tetrazole. The 1H- and 2H- isomers aretautomers,with the equilibrium lying on the side of 1H-tetrazole in the solid phase.[3][4][5]In the gas phase, 2H-tetrazole dominates.[4][6][7]These isomers can be regarded asaromatic,with 6 π-electrons, while the 5H-isomer is nonaromatic.
Phosphorus analogs do not have the same electronic nature, with 1H-tetraphosphole having a morepyramidalgeometry of the phosphorus at position 1. Instead, it is theanionictetraphospholides that are aromatic.[8]
Stronglyinductively electron-withdrawingfunctional groups attached to a tetrazole may stabilize a tautomeric ring-opening equilibrium with an azidoimine form.[9]
Synthesis
[edit]1H-Tetrazole was first prepared by the reaction of anhydroushydrazoic acidandhydrogen cyanideunder pressure. Treatment of organicnitrileswithsodium azidein the presence ofiodineor silica-supportedsodium bisulfateas a heterogeneouscatalystenables an advantageous synthesis of 5-substituted 1H-tetrazoles. Another method is the deamination of5-aminotetrazole,which can be commercially obtained or prepared in turn fromaminoguanidine.[10][11]
2-Aryl-2H-tetrazoles are synthesized by a [3+2]cycloadditionreaction between an aryldiazoniumandtrimethylsilyldiazomethane.[12]
Uses
[edit]There are several pharmaceutical agents which are tetrazoles, including severalcephalosporin-class antibiotics. Tetrazoles can act asbioisosteresforcarboxylate groupsbecause they have similar pKa and are deprotonated at physiological pH.AngiotensinII receptor blockers — such aslosartanandcandesartan,often are tetrazoles. A well-known tetrazole is dimethyl thiazolyl diphenyl tetrazolium bromide (MTT). This tetrazole is used in theMTT assayto quantify therespiratory activityof live cellsculture,although it generally kills the cells in the process. Some tetrazoles can also be used in DNA assays.[13]Studies suggest VT-1161 and VT-1129 are a potential potent antifungal drugs as they disturbs fungal enzymatic function but not human enzymes.[14][15]
Some tetrazolederivativeswith high energy have been investigated as high performanceexplosivesas a replacement forTNTand also for use in high performancesolid rocket propellantformulations.[16][17]These include theazidotetrazolatesalts of nitrogen bases.
Other tetrazoles are used for their explosive or combustive properties, such as tetrazole itself and5-aminotetrazole,which are sometimes used as a component ofgas generatorsinautomobileairbags.Tetrazole based energetic materials produce high-temperature, non-toxic reaction products such as water and nitrogen gas,[18]and have a high burn rate and relative stability,[19]all of which are desirable properties. The delocalization energy in tetrazole is 209 kJ/mol.
1H-Tetrazole and 5-(benzylthio)-1H-tetrazole (BTT) are widely used as acidic activators of the coupling reaction inoligonucleotide synthesis.[20]
2-Tetrazoles can undergo controlledthermal decompositionto form highly reactivenitrilimines.[21][22]These can in turn undergo a variety of1,3-dipolar cycloadditionreactions.[23]

Related heterocycles
[edit]- Triazoles,analogs with three nitrogen atoms
- Pentazole,the analog with five nitrogen atoms (strictly speaking, an inorganic homocycle, not a heterocycle)
- Oxatetrazole
- Thiatetrazole
References
[edit]- ^Satchell, Jacqueline F.; Smith, Brian J. (2002). "Calculation of aqueous dissociation constants of 1,2,4-triazole and tetrazole: A comparison of solvation models".Phys. Chem. Chem. Phys.4(18): 4314–4318.Bibcode:2002PCCP....4.4314S.doi:10.1039/b203118c.
- ^Mihina, Joseph S.; Herbst, Robert M. (1950). "The Reaction of Nitriles with Hydrazoic Acid: Synthesis of Monosubstituted Tetrazoles".J. Org. Chem.15(5): 1082–1092.doi:10.1021/jo01151a027.
- ^Goddard, R.; Heinemann, O.; Krüger, C. (1997-05-15)."α-1H-1,2,3,4-Tetrazole".Acta Crystallographica Section C.53(5): 590–592.Bibcode:1997AcCrC..53..590G.doi:10.1107/S0108270197000772.ISSN0108-2701.
- ^abKiselev, Vitaly G.; Cheblakov, Pavel B.; Gritsan, Nina P. (2011-03-10)."Tautomerism and Thermal Decomposition of Tetrazole: High-Level ab Initio Study".The Journal of Physical Chemistry A.115(9): 1743–1753.Bibcode:2011JPCA..115.1743K.doi:10.1021/jp112374t.ISSN1089-5639.PMID21322546.
- ^Razynska, A.; Tempczyk, A.; Malinski, E.; Szafranek, J.; Grzonka, Z.; Hermann, P.: inJ. Chem. Soc.Perkin Trans. 2 1983, 379.
- ^Wong, Ming Wah; Leung-Toung, Regis; Wentrup, Curt (1993-03-01)."Tautomeric equilibrium and hydrogen shifts of tetrazole in the gas phase and in solution".Journal of the American Chemical Society.115(6): 2465–2472.doi:10.1021/ja00059a048.ISSN0002-7863.
- ^Rażyńska, Anna; Tempczyk, Anna; Maliński, Edmund; Szafranek, Janusz; Grzonka, Zbigniew; Hermann, Peter (1983-01-01)."Application of mass spectrometry to the study of prototropic equilibria in 5-substituted tetrazoles in the gas phase; experimental evidence and theoretical considerations".Journal of the Chemical Society, Perkin Transactions 2(3): 379–383.doi:10.1039/P29830000379.ISSN1364-5471.
- ^Collier, S. J. (2004). "Product Class 24: Tetraphospholes". In Storr, R. C.; Gilchrist, T. L. (eds.).Science of Synthesis.Vol. 13: Category 2, Hetarenes and Related Ring Systems. Thieme.doi:10.1055/sos-SD-013-01194.ISBN978-3-13-112281-0.
- ^Burke, Luke A. (25 April 1983). "Possible cause for 5-trichloromethyltetrazole explosion" (letter to the editor),Chemical & Engineering News.p. 2.doi:10.1021/cen-v061n017.p002;but seeBeck, Wolfgang and Geisenberger, Josef (5 Mar 1984). "5-Trichloromethyltetrazole",Ibid.p. 39.doi:10.1021/cen-v062n010.p002,which indicates that the trichloromethyl derivative does not exhibit such an equilibrium.
- ^Henry, Ronald A.; Finnegan, William G. (1954-01-01)."An Improved Procedure for the Deamination of 5-Aminotetrazole".Journal of the American Chemical Society.76(1): 290–291.doi:10.1021/ja01630a086.ISSN0002-7863.
- ^Kurzer, F.; Godfrey, L. E. A. (1963)."Syntheses of Heterocyclic Compounds from Aminoguanidine".Angewandte Chemie International Edition in English.2(8): 459–476.doi:10.1002/anie.196304591.ISSN1521-3773.
- ^Patouret, Remi; Kamenecka, Theodore M. (2016-04-06)."Synthesis of 2-aryl-2H-tetrazoles via a regioselective [3+2] cycloaddition reaction".Tetrahedron Letters.57(14): 1597–1599.doi:10.1016/j.tetlet.2016.02.102.PMC4810784.PMID27041776.
- ^S Berner; K Mühlegger & H Seliger (Feb 11, 1989)."Studies on the role of tetrazole in the activation of phosphoramidites".Nucleic Acids Res.17(3): 853–864.doi:10.1093/nar/17.3.853.PMC331708.PMID2922273.
- ^Warrilow, A. G. S.;Hull, C. M.;Parker, J. E.; Garvey, E. P.; Hoekstra, W. J.; Moore, W. R.; Schotzinger, R. J.; Kelly, D. E.; Kelly, S. L. (December 2014)."The Clinical Candidate VT-1161 Is a Highly Potent Inhibitor of Candida albicans CYP51 but Fails To Bind the Human Enzyme".Antimicrobial Agents and Chemotherapy.58(12): 7121–7127.doi:10.1128/AAC.03707-14.PMC4249504.PMID25224009.
- ^Lockhart, Shawn R.; Fothergill, Annette W.; Iqbal, Naureen; Bolden, Carol B.; Grossman, Nina T.; Garvey, Edward P.; Brand, Stephen R.; Hoekstra, William J.; Schotzinger, Robert J.; Ottinger, Elizabeth; Patterson, Thomas F.; Wiederhold, Nathan P. (April 2016)."The Investigational Fungal Cyp51 Inhibitor VT-1129 Demonstrates Potent Activity against Cryptococcus neoformans and Cryptococcus gattii".Antimicrobial Agents and Chemotherapy.60(4): 2528–2531.doi:10.1128/AAC.02770-15.PMC4808209.PMID26787697.
- ^"Greener explosives show promise".Chemistry World. 2 October 2008.
- ^Niko Fischer; Konstantin Karaghiosoff;Thomas M. Klapötke;Jörg Stierstorfer (April 2010). "New Energetic Materials featuring Tetrazoles and Nitramines – Synthesis, Characterization and Properties".Zeitschrift für Anorganische und Allgemeine Chemie.636(5): 735–749.doi:10.1002/zaac.200900521.
- ^Tore Brinck, Thomas M. Klapötke and Jörg Stierstorfer (2014). "Energetic Tetrazole N-oxides".Energetic Tetrazole N -oxides.pp. 133–178.doi:10.1002/9781118676448.ch06.ISBN9781118676448.
{{cite book}}:|journal=ignored (help) - ^Nicholas Piekiel & Michael R. Zachariah (2012). "Decomposition of Aminotetrazole Based Energetic Materials under High Heating Rate Conditions".J. Phys. Chem. A.116(6): 1519–1526.Bibcode:2012JPCA..116.1519P.doi:10.1021/jp203957t.PMID22214278.
- ^Xia Wei (May 6, 2013). "Coupling activators for the oligonucleotide synthesis via phosphoramidite approach".Tetrahedron.69(18): 3615–3637.doi:10.1016/j.tet.2013.03.001.
- ^Huisgen, Rolf; Seidel, Michael; Sauer, Juergen; McFarland, James; Wallbillich, Guenter (June 1959). "Communications: The Formation of Nitrile Imines in the Thermal Breakdown of 2,5-Disubstituted Tetrazoles".The Journal of Organic Chemistry.24(6): 892–893.doi:10.1021/jo01088a034.
- ^Bertrand, Guy; Wentrup, Curt (17 March 1994). "Nitrile Imines: From Matrix Characterization to Stable Compounds".Angewandte Chemie International Edition in English.33(5): 527–545.doi:10.1002/anie.199405271.
- ^Huisgen, Rolf (October 1963). "1,3-Dipolar Cycloadditions. Past and Future".Angewandte Chemie International Edition in English.2(10): 565–598.doi:10.1002/anie.196305651.