T helper cell
TheT helper cells(Thcells), also known asCD4+cellsorCD4-positive cells,are a type ofT cellthat play an important role in theadaptive immune system.They aid the activity of other immune cells by releasingcytokines.They are considered essential inB cellantibody class switching,breakingcross-tolerancein dendritic cells, in the activation and growth ofcytotoxic T cells,and in maximizingbactericidalactivity ofphagocytessuch asmacrophagesandneutrophils.CD4+cells are mature Thcells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+cells determines susceptibility to a broad class ofautoimmune diseases.[1]
Structure and function[edit]
Thcells contain and release cytokines to aid other immune cells. Cytokines are small protein mediators that alter the behavior of target cells that expressreceptorsfor those cytokines. These cells help polarize the immune response depending on the nature of the immunological insult (for example; virus vs. extracellularbacteriumvs. intracellular bacterium vs.helminthvs. fungus vs. protist).[citation needed]
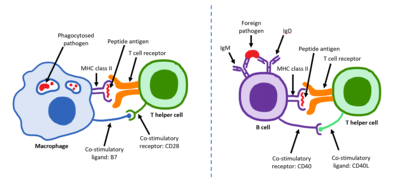
Mature Thcells express the surface proteinCD4and are referred to asCD4+T cells.CD4+T cells are generally treated as having a pre-defined role as helper T cells within theimmune system.For example, when anantigen-presenting celldisplays apeptideantigen onMHC class IIproteins, a CD4+cell will aid those cells through a combination of cell to cell interactions (e.g.CD40 (protein)andCD40L) and throughcytokines.
Thcells are not a monolithic immunological entity because they are diverse in terms of function and their interaction with partner cells. In general, mature naive T cells are stimulated by professional antigen presenting cells to acquire an effector module. These are defined by the presence of a lineage-determining (or lineage-specifying) transcription factor (also calledmaster regulator,though the term has been criticized for being too reductive).[2]The loss of function in a lineage specifying transcription factor results in the absence of the corresponding class of helper T cell which can be devastating for the health of the host.
Activation of naive helper T cells[edit]

FollowingDevelopmentin thethymus,these cells (termed recent thymic emigrants (RTE)) egress from the thymus and home tosecondary lymphoid organs(SLO;spleenandlymph nodes). Of note, only a very small minority of T cells egresses from the thymus (estimates commonly range from 1–5% but some experts feel even this is generous).[3]Maturation of RTE in SLO results in the generation of maturenaive T cells(naïve meaning they have never been exposed to theantigenthat they are programmed to respond to), but naive T cells now lack or havedownregulated(reduced) expression of the RTE-related surface markers, such asCD31,PTK7,Complement Receptor 1 and 2 (CR1,CR2) and the production ofinterleukin 8 (IL-8).[4][5]Like all T cells, they express theT cell receptor-CD3complex. The T cell receptor (TCR) consists of both constant and variable regions. The variable region determines what antigen the T cell can respond to. CD4+T cells have TCRs with an affinity forClass II MHC,and CD4 is involved in determining MHC affinity during maturation in thethymus.Class II MHC proteins are generally only found on the surface of professionalantigen-presenting cells(APCs). Professional antigen-presenting cells are primarilydendritic cells,macrophagesandB cells,although dendritic cells are the only cell group that expresses MHC Class IIconstitutively(at all times). Some APCs also bind native (or unprocessed) antigens to their surface, such asfollicular dendritic cells(these arenotthe same type of cells as thedendritic cellsof the immune system but rather have a non-hematopoietic origin, and in general lack MHC Class II, meaning they are not true professional antigen-presenting cells; however, follicular dendritic cells may acquire MHC Class II proteins via exosomes that become attached to them[6]). T cells requireantigens to be processedinto short fragments which formlinear epitopeson MHC Class II (in the case of helper T cells because they express CD4) or MHC class I (in the case ofcytotoxic T cellswhich expressCD8). MHC Class II binding pockets are flexible with respect to the length of the peptides they hold. Generally, there are 9 core amino acid residues with several flanking amino acids which form a length of about 12–16 amino acids total[7]but have been known to hold as many as 25 amino acids.[8]By comparison, MHC Class I proteins are usually 9-10 peptides long.[9]The activation of naive T cells is commonly explained in terms of the 3-signal model, elaborated upon below.[10]
Activation (signal 1)[edit]
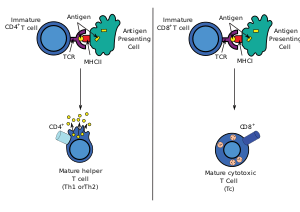
During an immune response,professional antigen-presenting cells(APCs)endocytoseantigens (typically bacteria or viruses), which undergoprocessing,then travel from the infection site to thelymph nodes.Typically, the APC responsible is a dendritic cell. If the antigen expresses appropriate molecular patterns (sometimes known as signal 0), it can induce maturation of the dendritic cell which results in enhanced expression of costimulatory molecules needed to activate T cells (see signal 2)[11]and MHC Class II.[12]Once at the lymph nodes, the APCs begin to present antigen peptides that are bound to Class II MHC, allowing CD4+T cells that express the specific TCRs against the peptide/MHC complex to activate.[citation needed]
When a Thcell encounters and recognizes the antigen on an APC, theTCR-CD3complex binds strongly to the peptide-MHC complex present on the surface of professional APCs.CD4,a co-receptor of the TCR complex, also binds to a different section of the MHC molecule. It is estimated that approximately 50 of these interactions are required for the activation of a helper T cell and assemblies known as microclusters have been observed forming between the TCR-CD3-CD4 complexes of the T cell and the MHC Class II proteins of the dendritic cell at the zone of contact. When these all come together, the CD4 is able to recruit a kinase calledLckwhich phosphorylatesimmunoreceptor tyrosine-based activation motifs(ITAMs) present on the CD3 gamma, delta, epsilon, and zeta chains. The proteinZAP-70can bind these phosphorylated ITAMs via itsSH2 domainand then itself becomes phosphorylated, wherein it orchestrates the downstream signaling required for T cell activation. Lck activation is controlled by the opposing actions ofCD45andCsk.[13]CD45 activates Lck by dephosphorylating a tyrosine in its C-terminal tail, while Csk phosphorylates Lck at that site. The loss of CD45 produces a form of SCID because failure to activate Lck prevents appropriate T cell signaling. Memory T cells also make use of this pathway and have higher levels of Lck expressed and the function of Csk is inhibited in these cells.[14]
The binding of the antigen-MHC to the TCR complex and CD4 may also help the APC and the Thcell adhere during Thcell activation, but the integrin proteinLFA-1on the T cell andICAMon the APC are the primary molecules of adhesion in this cell interaction.[citation needed]
It is unknown what role the relatively bulky extracellular region of CD45 plays during cell interactions, but CD45 has various isoforms that change in size depending on the Thcell's activation and maturation status. For example, CD45 shortens in length following Thactivation (CD45RA+to CD45RO+), but whether this change in length influences activation is unknown. It has been proposed that the larger CD45RA may decrease the accessibility of the T cell receptor for the antigen-MHC molecule, thereby necessitating an increase in the affinity (and specificity) of the T cell for activation. However, once the activation has occurred, CD45 shortens, allowing easier interactions and activation as an effector T helper cell.[citation needed]
Survival (signal 2)[edit]
Having received the first TCR/CD3 signal, the naïve T cell must activate a second independent biochemical pathway, known as Signal 2. This verification step is a protective measure to ensure that a T cell is responding to a foreign antigen. If this second signal is not present during initial antigen exposure, the T cell presumes that it is auto-reactive. This results in the cell becominganergic(anergy is generated from the unprotected biochemical changes of Signal 1). Anergic cells will not respond to any antigen in the future, even if both signals are present later on. These cells are generally believed to circulate throughout the body with no value until they undergoapoptosis.[15]
The second signal involves an interaction betweenCD28on the CD4+T cell and the proteinsCD80(B7.1) orCD86(B7.2) on the professional APCs. Both CD80 and CD86 activate the CD28 receptor. These proteins are also known asco-stimulatory molecules.[citation needed]
Although the verification stage is necessary for the activation of naïve helper T cells, the importance of this stage is best demonstrated during the similar activation mechanism of CD8+cytotoxic T cells.As naïve CD8+T cells have no true bias towards foreign sources, these T cells must rely on the activation of CD28 for confirmation that they recognize a foreign antigen (as CD80/CD86 is only expressed by active APC's). CD28 plays an important role in decreasing the risk of T cell auto-immunity against host antigens.[citation needed]
Once the naïve T cell has both pathways activated, the biochemical changes induced by Signal 1 are altered, allowing the cell to activate instead of undergoing anergy. The second signal is then obsolete; only the first signal is necessary for future activation. This is also true for memory T cells, which is one example oflearned immunity.Faster responses occur upon reinfection because memory T cells have already undergone confirmation and can produce effector cells much sooner.[citation needed]
Differentiation (signal 3)[edit]
Once the two-signal activation is complete the T helper cell (Th) then allows itself toproliferate.It achieves this by releasing a potent T cell growth factor calledinterleukin 2(IL-2) which acts upon itself in anautocrinefashion. Activated T cells also produce the alpha sub-unit of theIL-2 receptor(CD25or IL-2R), enabling a fully functional receptor that can bind with IL-2, which in turn activates the T cell's proliferation pathways.[citation needed]
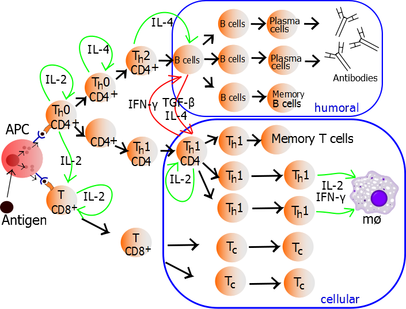
Theautocrineorparacrinesecretion of IL-2 can bind to that same Thcell or neighboring Th's via the IL-2R thus driving proliferation and clonal expansion. The Thcells receiving both signals of activation and proliferation will then become Th0 (T helper 0) cells that secrete IL-2,IL-4andinterferon gamma(IFN-γ). The Th0 cells will then differentiate into Th1 or Th2 cells depending oncytokineenvironment. IFN-γ drives Th1 cell production whileIL-10and IL-4 inhibit Th1 cell production. Conversely, IL-4 drives Th2 cell production and IFN-γ inhibits Th2 cells. These cytokines arepleiotropicand carry out many other functions of the immune response.[citation needed]
Effector function[edit]
In 1991, three groups reported discovering CD154, which is the molecular basis of T cell helper function.Seth LedermanatColumbia Universitygenerated a murine monoclonal antibody, 5c8 that inhibited contact-dependent T cell helper function in human cells which characterized the 32 kDa surface protein transiently expressed on CD4+T cells.[16]Richard Armitage atImmunexcloned a cDNA encoding CD154 by screening an expression library with CD40-Ig.[17]Randolph Noelle atDartmouth Medical Schoolgenerated an antibody that bound a 39 kDa protein on murine T cells and inhibited helper function.[18]
Determination of the effector T cell response[edit]
Helper T cells are capable of influencing a variety of immune cells, and the T cell response generated (including theextracellularsignals such ascytokines) can be essential for a successful outcome from infection. In order to be effective, helper T cells must determine which cytokines will allow the immune system to be most useful or beneficial for the host. Understanding exactly how helper T cells respond to immune challenges is currently of major interest inimmunology,because such knowledge may be very useful in the treatment of disease and in increasing the effectiveness ofvaccination.[citation needed]
Th1/Th2 model[edit]
Proliferating helper T cells that develop into effector T cells differentiate into two major subtypes of cells known as Th1 and Th2 cells (also known as Type 1 and Type 2 helper T cells, respectively).
Th1 helper cells lead to an increasedcell-mediated response(primarily bymacrophagesandcytotoxic T cells),[19]typically against intracellular bacteria and protozoa. They are triggered by the polarising cytokine IL-12 and their effector cytokines are IFN-γ and IL-2. The main effector cells of Th1 immunity are macrophages as well as CD8 T cells, IgG B cells, and IFN-γ CD4 T cells. The key Th1 transcription factors are STAT4 and T-bet. IFN-γ secreted by CD4 T cells can activate macrophages to phagocytose and digest intracellular bacteria and protozoa. In addition, IFN-γ can activateiNOS(inducible nitric oxide synthase) to produce nitric oxide free radicals to directly kill intracellular bacteria and protozoa. Th1 overactivation against autoantigens will causeType IVor delayed-type hypersensitivity reaction.Tuberculin reactionand Type 1 diabetes belong to this category of autoimmunity.[20]
Th2 helper cells lead to ahumoral immune response,[19]typically against extracellular parasites such ashelminths.They are triggered by the polarising cytokines IL-4 and IL-2, and their effector cytokines are IL-4, IL-5, IL-9, IL-10, IL-13 and IL-25. The main effector cells are eosinophils, basophils, and mast cells as well as B cells, and IL-4/IL-5 CD4 T cells. The key Th2 transcription factors areSTAT6andGATA3.[21]IL-4 is the positive feedback cytokine for Th2 cells differentiation. Besides, IL-4 stimulates B-cells to produce IgE antibodies, which in turn stimulate mast cells to releasehistamine,serotonin,and leukotriene to cause broncho-constriction, intestinal peristalsis, gastric fluid acidification to expel helminths. IL-5 from CD4 T cells will activate eosinophils to attack helminths. IL-10 suppresses Th1 cells differentiation and function of dendritic cells. Th2 overactivation against antigen will causeType I hypersensitivitywhich is an allergic reaction mediated by IgE. Allergic rhinitis, atopic dermatitis, and asthma belong to this category of overactivation.[20]In addition to expressing different cytokines, Th2 cells also differ from Th1 cells in their cell surface glycans (oligosaccharides), which makes them less susceptible to some inducers of cell death.[22][23]
| Type 1/ Th1 | Type 2/ Th2[20] | |
|---|---|---|
| Main partner cell type | Macrophage,CD8+T cell | B-cell,eosinophil,mast cell |
| Cytokines produced | Interferon gamma(IFNγ) andTNF-β.Interleukin 2andinterleukin 10production has been reported in activated Th1 cell.[25] | Interleukin 4,interleukin 5,interleukin 6,interleukin 9,interleukin 10,interleukin 13 |
| Immune stimulation promoted | Cellular immune system.Maximizes the killing efficacy of themacrophagesand the proliferation of cytotoxicCD8+T cells. Also promotes the production of IgG, an opsonizing antibody. | Humoral immune system.StimulatesB-cellsinto proliferation, to induce B-cellantibody class switching,and to increase neutralizingantibodyproduction (IgG, IgM and IgA as well as IgE antibodies). |
| Other functions | The Type 1 cytokineIFNγincreases the production ofinterleukin 12by dendritic cells and macrophages, and via positive feedback, IL-12 stimulates the production ofIFNγin helper T cells, thereby promoting the Th1 profile. IFNγ also inhibits the production of cytokines such asinterleukin 4,an important cytokine associated with the Type 2 response, and thus it also acts to preserve its own response. | The Type 2 response promotes its own profile using two different cytokines.Interleukin 4acts on helper T cells to promote the production of Th2 cytokines (including itself; it is auto-regulatory), whileinterleukin 10(IL-10) inhibits a variety of cytokines includinginterleukin 2andIFNγin helper T cells and IL-12 in dendritic cells and macrophages. The combined action of these two cytokines suggests that once the T cell has decided to produce these cytokines, that decision is preserved (and also encourages other T cells to do the same). |
While we know about the types of cytokine patterns helper T cells tend to produce, we understand less about how the patterns themselves are decided. Various evidence suggests that the type of APC presenting the antigen to the T cell has a major influence on its profile. Other evidence suggests that the concentration of antigen presented to the T cell during primary activation influences its choice. The presence of some cytokines (such as the ones mentioned above) will also influence the response that will eventually be generated, but our understanding is nowhere near complete.
Th17 helper cells[edit]
Th17 helper cellsare a subset of T helper cells developmentally distinct from Th1 and Th2 lineages. Th17 cells produceinterleukin 17(IL-17), a pro-inflammatory substance, as well asinterleukins 21and22.[26]This means that Th17 cells are especially good at fighting extracellular pathogens[26]and fungi, particularly during mucocutaneous immunity againstCandidaspp.[27]
THαβ helper cells[edit]
THαβ helper cells provide the host immunity against viruses. Their differentiation is triggered by IFN α/β orIL-10.Their key effector cytokine is IL-10. Their main effector cells areNK cellsas well as CD8 T cells, IgG B cells, and IL-10 CD4 T cells. The key THαβ transcription factors areSTAT1and STAT3 as well as IRFs. IL-10 from CD4 T cells activate NK cells'ADCCto apoptose virus-infected cells and to induce host as well as viral DNA fragmentation. IFN alpha/beta can suppress transcription to avoid virus replication and transmission. Overactivation of THαβ against autoantigen will cause type 2 antibody-dependent cytotoxic hypersensitivity.Myasthenia gravisorGraves' diseasebelong to this category.[28]
Limitations to the Th1/Th2 model[edit]
The interactions between cytokines from the Th1/Th2 model can be more complicated in some animals. For example, the Th2 cytokineIL-10inhibits cytokine production of both Thsubsets in humans. Human IL-10 (hIL-10) suppresses the proliferation and cytokine production of all T cells and the activity of macrophages, but continues to stimulateplasma cells,ensuring that antibody production still occurs. As such, hIL-10 is not believed to truly promote the Th2 response in humans, but acts to prevent over-stimulation of helper T cells while still maximising the production ofantibodies.[citation needed]
There are also other types of T cells that can influence the expression and activation of helper T cells, such as naturalregulatory T cells,along with less common cytokine profiles such as theTh3subset of helper T cells. Terms such as "regulatory" and "suppression" have become ambiguous after the discovery that helper CD4+T cells are also capable of regulating (and suppressing) their own responses outside of dedicated regulatory T cells.[citation needed]
One major difference between regulatory T cells and effector T cells is that regulatory T cells typically serve to modulate and deactivate the immune response, while effector T cell groups usually begin with immune-promoting cytokines and then switch to inhibitory cytokines later in their life cycle. The latter is a feature of Th3 cells, which transform into a regulatory subset after its initial activation and cytokine production.[citation needed]
Bothregulatory T cellsandTh3 cellsproduce the cytokinetransforming growth factor-beta(TGF-β) and IL-10. Both cytokines are inhibitory to helper T cells; TGF-β suppresses the activity of most of the immune system. There is evidence to suggest that TGF-β may not suppress activated Th2 cells as effectively as it might suppress naive cells, but it is not typically considered a Th2 cytokine.[citation needed]
The novel characterisation of another T helper subtype,T helper 17 cells(Th17)[29]has cast further doubt on the basic Th1/Th2 model. TheseIL-17producing cells were initially described as a pathogenic population implicated in autoimmunity but are now thought to have their own distinct effector and regulatory functions. Of note, some evidence suggest that functional plasticity is an intrinsic capacity of T helper cells. Indeed, a study in mice demonstrated that Th17 cells transform into Th1 cellsin vivo.[30]A subsequent study furthermore showed that extensive T helper cell plasticity is also prominent in humans.[31]
Many of the cytokines in this article are also expressed by other immune cells (see individual cytokines for details), and it is becoming clear that while the original Th1/Th2 model is enlightening and gives insight into the functions of helper T cells, it is far too simple to define its entire role or actions. Some immunologists question the model completely, as somein vivostudies suggest that individual helper T cells usually do not match the specific cytokine profiles of the Thmodel, and many cells express cytokines from both profiles.[32]That said, the Thmodel has still played an important part in developing our understanding of the roles and behaviour of helper T cells and the cytokines they produce during an immune response.
Studies by Stockinger et al. revealed that another T helper subset may exist.Th9 cellsare claimed to be an IL9 (interleukin 9)–producing T cell subset focused on defendinghelminthinfections.[33]
Memory T cell[edit]
Historically, memory T cells were thought to belong to either the effector or central memory subtypes, each with their own distinguishing set of cell surface markers.[34]Central memory T cells reside in the lymph nodes while effector memory T cells lack theC-C chemokine receptor type 7(CCR7) andL-selectin(CD62L) receptors, which prevents them from trafficking to the lymph nodes.
Additional populations of memory T cells are now known to exist. These include tissue-resident memory T (Trm) cells and virtual memory T cells.[35]The single unifying theme for all memory T cell subtypes is that they are long-lived and can expand quickly to large numbers of effector T cells upon encountering their cognate antigen. By this mechanism they provide the immune system with "memory" against previously encountered pathogens.
Role in disease[edit]
Considering the diverse and important role helper T cells play in the immune system, it is not surprising that these cells often influence the immune response against disease. They also occasionally generate non-beneficial responses. Very rarely, the helper T cell response could lead to the death of the host.[citation needed]
Antitumor immunity[edit]
Hypersensitivity[edit]
The immune system must achieve a balance of sensitivity in order to respond to foreign antigens without responding to the antigens of the host itself. When the immune system responds to very low levels of antigen that it usually shouldn't respond to, ahypersensitivityresponse occurs. Hypersensitivity is believed to be the cause ofallergyand someauto-immune disease.
Hypersensitivity reactions can be divided into four types:
- Type 1 hypersensitivityincludes common immune disorders such asasthma,allergic rhinitis(hay fever),eczema,urticaria(hives) andanaphylaxis.These reactions all involveIgEantibodies,which require a Th2 response during helper T cell development. Preventive treatments, such ascorticosteroidsandmontelukast,focus on suppressing mast cells or other allergic cells; T cells do not play a primary role during the actual inflammatory response. It's important to note that the numeral allocation of hypersensitivity "types" does not correlate (and is completely unrelated) to the "response" in the Thmodel.
- Type 2andType 3 hypersensitivityboth involve complications from auto-immune or low affinity antibodies. In both of these reactions, T cells may play an accomplice role in generating these auto-specific antibodies, although some of these reactions under Type 2 hypersensitivity would be considered normal in a healthy immune system (for example,Rhesus factor reactionsduring child-birth is a normal immune response against child antigens). The understanding of the role of helper T cells in these responses is limited but it is generally thought that Th2 cytokines would promote such disorders. For example, studies have suggested thatlupus(SLE) and other auto-immune diseases of similar nature can be linked to the production of Th2 cytokines.
- Type 4 hypersensitivity,also known asdelayed type hypersensitivity,are caused via the over-stimulation of immune cells, commonlylymphocytesandmacrophages,resulting in chronicinflammationand cytokine release. Antibodies do not play a direct role in this allergy type. T cells play an important role in this hypersensitivity, as they activate against the stimulus itself and promote the activation of other cells; particularly macrophages via Th1 cytokines.
Other cellular hypersensitivities includecytotoxic T cellmediatedauto-immune disease,and a similar phenomenon;transplant rejection.Helper T cells are required to fuel the development of these diseases. In order to create sufficientauto-reactivekiller T cells,interleukin-2must be produced, and this is supplied by CD4+T cells. CD4+T cells can also stimulate cells such asnatural killer cellsand macrophages via cytokines such asinterferon-gamma,encouraging these cytotoxic cells to kill host cells in certain circumstances.
The mechanism that killer T cells use during auto-immunity is almost identical to their response againstviruses,and some viruses have been accused of causing auto-immune diseases such asType 1 diabetes mellitus.Cellular auto-immune disease occurs because the host antigen recognition systems fail, and theimmune systembelieves, by mistake, that a host antigen is foreign. As a result, the CD8+T cells treat the host cell presenting that antigen as infected, and go on to destroy all host cells (or in the case of transplant rejection, transplant organ) that express that antigen.
Some of this section is a simplification. Many auto-immune diseases are more complex. A well-known example isrheumatoid arthritis,where both antibodies and immune cells are known to play a role in the pathology. Generally the immunology of most auto-immune diseases is not well understood.
HIV infection[edit]
Perhaps the best example of the importance ofCD4+T cells is demonstrated withhuman immunodeficiency virus(HIV) infection. HIV mainly targets lymphoid CD4+T cells, but can infect other cells that express CD4 such asmacrophagesanddendritic cells(both groups express CD4 at low levels).[citation needed]
It has been proposed that during the non-symptomatic phase of HIV infection, the virus has a relatively low affinity towards T cells (and has a higher affinity for macrophages), resulting in a slow kill rate of CD4+T cells by the immune system.[citation needed]This is initially compensated for via the production of new helper T cells from thethymus(originally from thebone marrow). Once the virus becomeslymphotropic(or T-tropic) however, it begins to infect CD4+T cells far more efficiently (likely due to a change in theco-receptorsit binds to during infection), and the immune system is overwhelmed. Studies suggest that only ~5% of the lymphoid-derived CD4 T cells targeted by HIV are permissive and become productively infected with the virus. More than 95% of the CD4 T cells that die are resting and are unable to support productive infection. These cells undergo abortive infection with HIV.[36]Cell death is triggered when the host cell detects HIV foreign DNA intermediates and initiates a suicidal death pathway in an attempt to protect the host, leading tocaspase-1activation ininflammasomes,thus causingpyroptosis(a highly inflammatory form of programmed cell death).[37][38][39]
At this point chronic inflammation ensues, and functional CD4+T cell levels begin to decrease, eventually to a point where the CD4+T cell population is too small to recognize the full range ofantigensthat could potentially be detected. The depletion of CD4 T cells and the development of chronic inflammation are signature processes in HIV pathogenesis that propel progression to acquired immune deficiency syndrome (AIDS). CD4 T cell depleted to the cell count of less than 200cell/μL in blood during AIDS allows variouspathogensto escape T cell recognition, thus allowingopportunistic infectionsthat would normally elicit a helper T cell response to bypass the immune system.[40]While these complete bypass situations only occur when the helper T cell response is absolutely necessary for infection clearance, most infections increase in severity and/or duration because the immune system's helper T cells provide less efficient immune response.
Two components of the immune system are particularly affected in AIDS, due to its CD4+T cell dependency:
- CD8+T cells are not stimulated as effectively during the AIDS stage of HIV infection, making AIDS patients very susceptible to most viruses, including HIV itself. This decline in killing of CD4+T cells results in the virus being produced for a longer period (the infected CD4+T cells are not killed as quickly), increasing the proliferation of the virus, and accelerating the development of the disease.
- Antibody class switchingdeclines significantly once helper T cell function fails. The immune system loses its ability to improve the affinity of their antibodies, and are unable to generate B cells that can produce antibody groups such asIgGandIgA.These effects are primarily due to the loss of any helper T cell that can interact with the B lymphocyte correctly. Another symptom of AIDS is the reduction in antibody levels due to a decrease in Th2 cytokines (and less interactions by helper T cells). All of these complications result in an increased susceptibility to aggressive bacterial infections, especially in areas of the body not accessible byIgMantibodies.
If the patient does not respond to (or does not receive)HIV treatmentthey will succumb usually to either cancers or infections; the immune system finally reaches a point where it is no longer coordinated or stimulated enough to deal with the disease.
Inhibition of CD4 T-cell expansion during HIV infection may occur due to microbial translocation in an IL-10-dependent way. Triggering PD-1 expressed on activated monocytes by its ligand PD-L1, induces IL-10 production which inhibits CD4 T-cell function.[41]
COVID-19[edit]
Incoronavirus disease 2019(COVID-19)B cell,natural killer cell,and totallymphocytecounts decline, but both CD4+and CD8+cells decline to a far greater extent.[42]Indicating thatSARS-Cov-2attacks the CD4+cells during infection. Low CD4+predicted greater likelihood ofintensive care unitadmission, and CD4+cell count was the only parameter that predicted length of time for viralRNAclearance.[42]Despite the reduced levels of CD4+,COVID-19 patients with severe disease had higher levels of Th1 CD4+cells than patients with moderate disease.[43]
See also[edit]
- CD4+/CD8+ratio
- CD4+T cells and antitumor immunity
- CD8+T cells
- Cancer vaccine targeting CD4+T cells
- List of distinct cell types in the adult human body
References[edit]
- ^Burren OS, Rubio García A, Javierre BM, Rainbow DB, Cairns J, Cooper NJ, et al. (September 2017)."Chromosome contacts in activated T cells identify autoimmune disease candidate genes".Genome Biology.18(1): 165.doi:10.1186/s13059-017-1285-0.PMC5584004.PMID28870212.
- ^Oestreich KJ, Weinmann AS (November 2012)."Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors".Nature Reviews. Immunology.12(11): 799–804.doi:10.1038/nri3321.PMC3584691.PMID23059426.
- ^Fink PJ (2013-03-21). "The biology of recent thymic emigrants".Annual Review of Immunology.31(1): 31–50.doi:10.1146/annurev-immunol-032712-100010.PMID23121398.
- ^van den Broek T, Borghans JA, van Wijk F (June 2018). "The full spectrum of human naive T cells".Nature Reviews. Immunology.18(6): 363–373.doi:10.1038/s41577-018-0001-y.PMID29520044.S2CID3736563.
- ^van den Broek T, Delemarre EM, Janssen WJ, Nievelstein RA, Broen JC, Tesselaar K, et al. (March 2016)."Neonatal thymectomy reveals differentiation and plasticity within human naive T cells".The Journal of Clinical Investigation.126(3): 1126–1136.doi:10.1172/JCI84997.PMC4767338.PMID26901814.
- ^Roche PA, Furuta K (April 2015)."The ins and outs of MHC class II-mediated antigen processing and presentation".Nature Reviews. Immunology.15(4): 203–216.doi:10.1038/nri3818.PMC6314495.PMID25720354.
- ^Unanue ER, Turk V, Neefjes J (May 2016). "Variations in MHC Class II Antigen Processing and Presentation in Health and Disease".Annual Review of Immunology.34(1): 265–297.doi:10.1146/annurev-immunol-041015-055420.PMID26907214.
- ^Wieczorek M, Abualrous ET, Sticht J, Álvaro-Benito M, Stolzenberg S, Noé F, Freund C (2017-03-17)."Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation".Frontiers in Immunology.8:292.doi:10.3389/fimmu.2017.00292.PMC5355494.PMID28367149.
- ^Trolle T, McMurtrey CP, Sidney J, Bardet W, Osborn SC, Kaever T, et al. (February 2016)."The Length Distribution of Class I-Restricted T Cell Epitopes Is Determined by Both Peptide Supply and MHC Allele-Specific Binding Preference".Journal of Immunology.196(4): 1480–1487.doi:10.4049/jimmunol.1501721.PMC4744552.PMID26783342.
- ^Murphy K (2017).Janeway's immunobiology.Garland Science.ISBN978-0-8153-4551-0.OCLC1020120603.
- ^Guy B (July 2007). "The perfect mix: recent progress in adjuvant research".Nature Reviews. Microbiology.5(7): 505–517.doi:10.1038/nrmicro1681.PMID17558426.S2CID25647540.
- ^Hammer GE, Ma A (2013-03-21)."Molecular control of steady-state dendritic cell maturation and immune homeostasis".Annual Review of Immunology.31(1): 743–791.doi:10.1146/annurev-immunol-020711-074929.PMC4091962.PMID23330953.
- ^Zamoyska R (September 2007)."Why is there so much CD45 on T cells?".Immunity.27(3): 421–423.doi:10.1016/j.immuni.2007.08.009.PMID17892852.
- ^Courtney AH, Shvets AA, Lu W, Griffante G, Mollenauer M, Horkova V, et al. (October 2019)."CD45 functions as a signaling gatekeeper in T cells".Science Signaling.12(604): eaaw8151.doi:10.1126/scisignal.aaw8151.PMC6948007.PMID31641081.
- ^Elmore S (June 2007)."Apoptosis: a review of programmed cell death".Toxicologic Pathology.35(4): 495–516.doi:10.1080/01926230701320337.PMC2117903.PMID17562483.
- ^Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L (April 1992)."Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help)".The Journal of Experimental Medicine.175(4): 1091–1101.doi:10.1084/jem.175.4.1091.PMC2119166.PMID1348081.
- ^Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, et al. (May 1992). "Molecular and biological characterization of a murine ligand for CD40".Nature.357(6373): 80–82.Bibcode:1992Natur.357...80A.doi:10.1038/357080a0.PMID1374165.S2CID4336943.
- ^Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A (July 1992)."A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells".Proceedings of the National Academy of Sciences of the United States of America.89(14): 6550–6554.Bibcode:1992PNAS...89.6550N.doi:10.1073/pnas.89.14.6550.PMC49539.PMID1378631.
- ^abBelizário JE, Brandão W, Rossato C, Peron JP (2016)."Thymic and Postthymic Regulation of Naïve CD4(+) T-Cell Lineage Fates in Humans and Mice Models".Mediators of Inflammation.2016:9523628.doi:10.1155/2016/9523628.PMC4904118.PMID27313405.
- ^abcZhu J, Paul WE (September 2008)."CD4 T cells: fates, functions, and faults".Blood.112(5): 1557–1569.doi:10.1182/blood-2008-05-078154.PMC2518872.PMID18725574.
- ^Wan YY (June 2014)."GATA3: a master of many trades in immune regulation".Trends in Immunology.35(6): 233–242.doi:10.1016/j.it.2014.04.002.PMC4045638.PMID24786134.
- ^Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, et al. (February 2015)."Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review".Journal of Autoimmunity.57(6): 1–13.doi:10.1016/j.jaut.2014.12.002.PMC4340844.PMID25578468.
- ^Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, et al. (August 2007). "Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death".Nature Immunology.8(8): 825–834.doi:10.1038/ni1482.PMID17589510.S2CID41286571.
- ^Rang HP, Dale MM, Riter JM, Moore PK (2003).Pharmacology.Edinburgh: Churchill Livingstone.ISBN978-0-443-07145-4.Page 223
- ^Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A (August 2009)."Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose".Immunity.31(2): 209–219.doi:10.1016/j.immuni.2009.05.012.PMC2791889.PMID19646904.
- ^abGuglani L, Khader SA (2010)."Th17 cytokines in mucosal immunity and inflammation".Current Opinion in HIV and AIDS.5(2): 120–127.doi:10.1097/COH.0b013e328335c2f6.PMC2892849.PMID20543588.
- ^Tangye SG, Puel A (2023). "The Th17/IL-17 Axis and Host Defense Against Fungal Infections".Journal of Allergy and Clinical Immunology: In Practice.11(6): 1624–1634.doi:10.1016/j.jaip.2023.04.015.PMID37116791.S2CID258380150.
- ^Hu W (2007).Microarray analysis of PBMC gene expression profiles after Plasmodium falciparum malarial infection(Ph.D. thesis). Johns Hopkins University.
- ^Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT (November 2005). "Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages".Nature Immunology.6(11): 1123–1132.doi:10.1038/ni1254.PMID16200070.S2CID11717696.
- ^Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. (March 2011)."Fate mapping of IL-17-producing T cells in inflammatory responses".Nature Immunology.12(3): 255–263.doi:10.1038/ni.1993.PMC3040235.PMID21278737.
- ^Larsen M, Arnaud L, Hié M, Parizot C, Dorgham K, Shoukry M, et al. (September 2011)."Multiparameter grouping delineates heterogeneous populations of human IL-17 and/or IL-22 T-cell producers that share antigen specificities with other T-cell subsets".European Journal of Immunology.41(9).UPMC Paris 06Institut National de la Santé et de la Recherche Médicale (Inserm) UMR-S 945: 2596–2605.doi:10.1002/eji.201041131.PMID21688259.S2CID24092508.
- ^Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ (June 2012)."Helper T cell diversity and plasticity".Current Opinion in Immunology.24(3): 297–302.doi:10.1016/j.coi.2012.01.014.PMC3383341.PMID22341735.
- ^Tato CM, Cua DJ (December 2008). "Alternative lifestyles of T cells".Nat Immunol.9(12): 1323–5.doi:10.1038/ni1208-1323.PMID19008928.S2CID6691974.
- ^Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (October 1999). "Two subsets of memory T lymphocytes with distinct homing potentials and effector functions".Nature.401(6754): 708–712.Bibcode:1999Natur.401..708S.doi:10.1038/44385.PMID10537110.S2CID4378970.
- ^Marusina AI, Ono Y, Merleev AA, Shimoda M, Ogawa H, Wang EA, et al. (February 2017)."CD4+virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity ".Journal of Autoimmunity.77(2): 76–88.doi:10.1016/j.jaut.2016.11.001.PMC6066671.PMID27894837.
- ^Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, et al. (November 2010)."Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue".Cell.143(5): 789–801.doi:10.1016/j.cell.2010.11.001.PMC3026834.PMID21111238.
- ^Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. (January 2014)."Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection".Nature.505(7484): 509–514.Bibcode:2014Natur.505..509D.doi:10.1038/nature12940.PMC4047036.PMID24356306.
- ^Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC (January 2014)."IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV".Science.343(6169): 428–432.Bibcode:2014Sci...343..428M.doi:10.1126/science.1243640.PMC3976200.PMID24356113.
- ^Zhang C, Song JW, Huang HH, Fan X, Huang L, Deng JN, et al. (March 2021)."NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1-infected patients".The Journal of Clinical Investigation.131(6).doi:10.1172/JCI138861.PMC7954596.PMID33720048.
- ^"CD4 Count".www.aids.gov.Archived fromthe originalon 2015-04-15.Retrieved2015-04-30.
- ^Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. (April 2010)."Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection".Nature Medicine.16(4): 452–459.doi:10.1038/nm.2106.PMC4229134.PMID20208540.
- ^abHuang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, et al. (August 2020)."Lymphocyte Subset Counts in COVID-19 Patients: A Meta-Analysis".Cytometry. Part A.97(8): 772–776.doi:10.1002/cyto.a.24172.PMC7323417.PMID32542842.
- ^Perlman S (August 2020)."COVID-19 poses a riddle for the immune system".Nature.584(7821): 345–346.doi:10.1038/d41586-020-02379-1.PMID32807916.
Further reading[edit]
- Doitsh G, Greene WC (March 2016)."Dissecting How CD4 T Cells Are Lost During HIV Infection".Cell Host & Microbe.19(3): 280–291.doi:10.1016/j.chom.2016.02.012.PMC4835240.PMID26962940.
- Kanno Y, Vahedi G, Hirahara K, Singleton K, O'Shea JJ (2012)."Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity".Annual Review of Immunology.30:707–731.doi:10.1146/annurev-immunol-020711-075058.PMC3314163.PMID22224760.
External links[edit]
- "T-cell Group".T-Cells.Cardiff University.
