Sodium thioantimoniate
| |
| Names | |
|---|---|
| IUPAC name
Sodium tetrathioantimonate(V)
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.208.207 |
| EC Number |
|
PubChemCID
|
|
| UNII |
|
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| Na3SbS4(anhydrous) Na3SbS4·9H2O(nonahydrate) | |
| Molar mass | 272.13g·mol−1(anhydrous) 434.27 g·mol−1(nonahydrate) |
| Appearance | Yellow crystals |
| Density | 1.806 g/cm3,solid |
| Melting point | 87 °C (189 °F; 360 K) |
| Hazards | |
| GHSlabelling: | |
 
| |
| Warning | |
| H302,H332,H411 | |
| P261,P264,P270,P271,P273,P301+P312,P304+P312,P304+P340,P312,P330,P391,P501 | |
| Related compounds | |
Othercations
|
Potassium thioantimoniate |
Related compounds
|
Antimony(III) sulfide |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
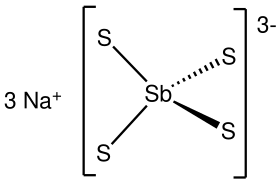
Sodium thioantimoniateorsodium tetrathioantimonate(V)is aninorganic compoundwith theformulaNa3SbS4.The nonahydrate of this chemical,Na3SbS4·9H2O,is known asSchlippe's salt,named after Johann Karl Friedrich von Schlippe (1799–1867). These compounds are examples ofsulfosalts.They were once of interest as species generated inqualitative inorganic analysis.
Structure
[edit]
The nonahydrate consists of the tetrahedral tetrathioantimonate(V) anionsSbS3−4and sodium cationsNa+,which arehydrated.The Sb-S distance is 2.33Å.[1][2]Related salts are known for different cations includingammoniumandpotassium.
The anhydrous salt is a polymer with tetrahedral Na and Sb sites.[3]
Preparation
[edit]Sodium tetrathioantimonate nonahydrate is prepared by the reaction of "antimony trisulfide",elementalsulfur,and aqueoussulfidesource.[4]
- 3 Na2S + 2 S + Sb2S3+ 18 H2O → 2 Na3SbS4·9H2O
The Na2S can be generated in situ by the reaction ofsodium hydroxideand S (co-generating sodium sulfate):
- Sb2S3+ 8 NaOH + 6 S → 2 Na3SbS4+ Na2SO4+ 4 H2O
Charcoal can also be used to reduce the sulfur.
The required antimony trisulfide is prepared by treatment of Sb(III) compounds with sulfide sources:
- 2 SbCl3+ 3 H2S → Sb2S3+ 6 HCl
Reactions
[edit]The hydrate dissolves in water to give the tetrahedralSbS3−4ion. The salt givesantimony pentasulfideupon acidification:
- 2 Na3SbS4+ 6 HCl → Sb2S5+ 6 NaCl + 3 H2S
Notes
[edit]- ^Krebs, B., "Thio- and Seleno Compounds of Main Group Elements - New Inorganic Oligomers and Polymers", Angewandte Chemie, 1983, volume 95, pages 113-34.
- ^K. Mereiter, A. Preisinger and H. Guth "Hydrogen bonds in Schlippe's salt: refinement of the crystal structures of Na3SbS4.9H2O by X-ray diffraction and Na3SbS4.9D2O by neutron diffraction at room temperature "Acta Crystallographica 1979, vol. B35, 19-25.doi:10.1107/S0567740879002442.
- ^H. A. Graf, H. Schäfer "Zur Strukturchemie der Alkalisalze der Tetrathiosäuren der Elemente der 5. Hauptgruppe (pages 67–80) Zeitschrift für Anorganische und Allgemeine Chemie 1976, vol. 425, p67-p80.doi:10.1002/zaac.19764250109
- ^P. W. Schenk (1963). "Arsenic, Antimony, Bismuth". In G. Brauer (ed.).Handbook of Preparative Inorganic Chemistry, 2nd Ed.Vol. 1pages=618. NY, NY: Academic Press.
References
[edit]- This article incorporates text from a publication now in thepublic domain:Chisholm, Hugh,ed. (1911). "Schlippe's Salt".Encyclopædia Britannica(11th ed.). Cambridge University Press.
