Threonine protease
| Threonine Protease | |||||||
|---|---|---|---|---|---|---|---|
 Crystal structure of humanproteasomealpha 1 | |||||||
| Identifiers | |||||||
| Symbol | Thr | ||||||
| |||||||
Threonine proteasesare a family ofproteolyticenzymesharbouring athreonine(Thr) residue within the active site. The prototype members of this class of enzymes are thecatalyticsubunits of theproteasome,however, theacyltransferasesconvergently evolved the sameactive sitegeometry andmechanism.
Mechanism
[edit]Threonine proteases use thesecondary alcoholof theirN-terminalthreonine as a nucleophile to perform catalysis.[1][2]The threonine must be N-terminal since the terminal amine of the same residue acts as ageneral baseby polarising anordered waterwhichdeprotonatesthe alcohol to increase its reactivity as a nucleophile.[3][4]
Catalysis takes place in two steps:
- Firstly thenucleophileattacks thesubstrateto form a covalentacyl-enzymeintermediate, releasing the first product.
- Secondly the intermediate ishydrolysedby water to regenerate the free enzyme and release the second product.
- In ornithineacyltransferase,instead of water, the substrateornithine(the acceptor) performs the second nucleophilic attack and so leaves with the acyl group.
Classification and evolution
[edit]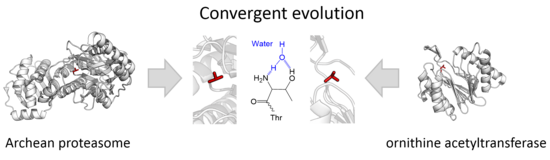
Five families belonging to two separatesuperfamiliesare currently recognised: the Ntn foldproteosomes[1](superfamily PB) and the DOM fold ornithineacyltransferases[2](superfamily PE). The two superfamilies represent two independent,convergent evolutionsof the same active site.[4][5]
| Superfamily | Threonine proteasefamilies | Examples |
|---|---|---|
| PB clan | T1, T2, T3, T6 | archaeanproteasome,beta component (Thermoplasma acidophilum) |
| PE clan | T5 | ornithineacetyltransferase(Saccharomyces cerevisiae) |
See also
[edit]- Protease
- Enzyme
- Proteolysis
- Catalytic triad
- Convergent evolution
- The Proteolysis Map
- Protease inhibitor (pharmacology)
- Protease inhibitor (biology)
- TopFIND- database of protease specificity, substrates, products and inhibitors
- MEROPS- database of protease evolutionary groups
References
[edit]- ^abBrannigan JA, Dodson G, Duggleby HJ, Moody PC, Smith JL, Tomchick DR, Murzin AG (November 1995). "A protein catalytic framework with an N-terminal nucleophile is capable of self-activation".Nature.378(6555): 416–9.Bibcode:1995Natur.378..416B.doi:10.1038/378416a0.PMID7477383.S2CID4277904.
- ^abCheng H, Grishin NV (July 2005)."DOM-fold: a structure with crossing loops found in DmpA, ornithine acetyltransferase, and molybdenum cofactor-binding domain".Protein Science.14(7): 1902–10.doi:10.1110/ps.051364905.PMC2253344.PMID15937278.
- ^Dodson G, Wlodawer A (September 1998). "Catalytic triads and their relatives".Trends in Biochemical Sciences.23(9): 347–52.doi:10.1016/S0968-0004(98)01254-7.PMID9787641.
- ^abEkici OD, Paetzel M, Dalbey RE (December 2008)."Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration".Protein Science.17(12): 2023–37.doi:10.1110/ps.035436.108.PMC2590910.PMID18824507.
- ^Buller AR, Townsend CA (February 2013)."Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad".Proceedings of the National Academy of Sciences of the United States of America.110(8): E653-61.Bibcode:2013PNAS..110E.653B.doi:10.1073/pnas.1221050110.PMC3581919.PMID23382230.
