Toll-like receptor
| Toll-like receptor | |
|---|---|
 The curvedleucine-rich repeatregion of toll-like receptors, represented here by TLR3 | |
| Identifiers | |
| Symbol | Toll-like receptor |
| Membranome | 7 |
| PIRSF037595 | |
Toll-like receptors(TLRs) are a class ofproteinsthat play a key role in theinnate immune system.They aresingle-spanningreceptorsusually expressed onsentinel cellssuch asmacrophagesanddendritic cells,that recognize structurally conserved molecules derived frommicrobes.Once these microbes have reached physical barriers such as the skin orintestinal tractmucosa,they are recognized by TLRs, which activateimmune cellresponses. The TLRs includeTLR1,TLR2,TLR3,TLR4,TLR5,TLR6,TLR7,TLR8,TLR9,TLR10,TLR11,TLR12,andTLR13.Humans lack genes for TLR11, TLR12 and TLR13[1]and mice lack a functional gene for TLR10.[2]The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on thecell membrane,whereas TLR3, TLR7, TLR8, and TLR9 are located inintracellularvesicles(because they are sensors ofnucleic acids).[3]
TLRs received their name from their similarity to the protein coded by thetoll gene.[4]
Function[edit]
The ability of the immune system to recognizemoleculesthat are broadly shared bypathogensis, in part, due to the presence ofimmune receptorscalled toll-like receptors (TLRs) that are expressed on themembranesofleukocytesincludingdendritic cells,macrophages,natural killer cells,cells of the adaptive immunityT cells,andB cells,and non-immune cells (epithelialandendothelial cells,andfibroblasts).[5]
The binding ofligands- either in the form of adjuvant used invaccinationsor in the form of invasive moieties during times of natural infection - to the TLR marks the keymolecularevents that ultimately lead toinnate immuneresponses and the development of antigen-specific acquired immunity.[6][7]
Upon activation, TLRs recruitadaptor proteins(proteins that mediate other protein-protein interactions) within thecytosolof theimmune cellto propagate the antigen-inducedsignal transduction pathway.These recruitedproteinsare then responsible for the subsequent activation of otherdownstreamproteins, includingprotein kinases(IKKi,IRAK1,IRAK4,andTBK1) that further amplify the signal and ultimately lead to the upregulation or suppression ofgenesthat orchestrateinflammatoryresponses and othertranscriptionalevents. Some of these events lead tocytokineproduction,proliferation,and survival, while others lead to greater adaptive immunity.[7]If the ligand is a bacterial factor, the pathogen might bephagocytosedand digested, and itsantigenspresented toCD4+ T cells. In the case of a viral factor, the infected cell may shut off its protein synthesis and may undergo programmed cell death (apoptosis). Immune cells that have detected a virus may also release anti-viral factors such asinterferons.
Toll-like receptors have also been shown to be an important link between innate and adaptive immunity through their presence indendritic cells.[8]Flagellin,a TLR5 ligand, induces cytokine secretion on interacting with TLR5 on human T cells.[8]
Superfamily[edit]

TLRs are a type ofpattern recognition receptor(PRR) and recognize molecules that are broadly shared bypathogensbut distinguishable from host molecules, collectively referred to aspathogen-associated molecular patterns(PAMPs). In addition to the recognition of exogenous PAMPs, TLRs can also bind to endogenousdamage-associated molecular patterns(DAMPs) such asheat shock proteins(HSPs) or plasma membrane constituents.[9]TLRs together with theInterleukin-1 receptorsform a receptorsuperfamily,known as the "interleukin-1 receptor / toll-like receptor superfamily"; all members of this family have in common a so-called TIR (toll-IL-1 receptor) domain.
Three subgroups of TIR domains exist. Proteins with subgroup 1 TIR domains are receptors forinterleukinsthat are produced bymacrophages,monocytes,anddendritic cellsand all have extracellularImmunoglobulin(Ig) domains. Proteins with subgroup 2 TIR domains are classical TLRs, and bind directly or indirectly to molecules of microbial origin. A third subgroup of proteins containing TIR domains consists ofadaptor proteinsthat are exclusivelycytosolicand mediate signaling from proteins of subgroups 1 and 2.
Extended family[edit]
This sectionis missing informationabout choanoflagellate TLR (pmid29848444).(December 2021) |
TLRs are present invertebratesas well asinvertebrates.Molecular building blocks of the TLRs are represented in bacteria and in plants, andplant pattern recognition receptorsare well known to be required for host defence against infection. The TLRs thus appear to be one of the most ancient, conserved components of theimmune system.
In recent years TLRs were identified also in the mammalian nervous system. Members of the TLR family were detected on glia, neurons and on neural progenitor cells in which they regulate cell-fate decision.[10]
It has been estimated that most mammalian species have between ten and fifteen types of toll-like receptors. Thirteen TLRs (named simply TLR1 to TLR13) have been identified in humans and mice together, and equivalent forms of many of these have been found in other mammalian species.[11][12][13]However, equivalents of certain TLR found in humans are not present in all mammals. For example, a gene coding for a protein analogous to TLR10 in humans is present inmice,but appears to have been damaged at some point in the past by aretrovirus.On the other hand, mice express TLRs 11, 12, and 13, none of which is represented in humans. Other mammals may express TLRs that are not found in humans. Other non-mammalian species may have TLRs distinct from mammals, as demonstrated by the anti-cell-wallTLR14,which is found in theTakifugupufferfish.[14]This may complicate the process of using experimental animals as models of human innate immunity.
Vertebrate TLRs are divided by similarity into the families of TLR 1/2/6/10/14/15, TLR 3, TLR 4, TLR 5, TLR 7/8/9, and TLR 11/12/13/16/21/22/23.[14]
TLRs inDrosophilaimmunity[edit]
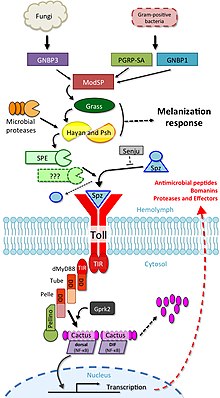
The involvement of toll signalling in immunity was first demonstrated in the fruit fly,Drosophila melanogaster.[19]Fruit flies have only innate immune responses allowing studies to avoid interference of adaptive immune mechanisms on signal transduction. The fly response to fungal or bacterial infection occurs through two distinct signalling cascades, one of which is the toll pathway and the other is theimmune deficiency pathway.The toll pathway is similar to mammalian TLR signalling, but unlike mammalian TLRs, toll is not activated directly by pathogen-associated molecular patterns (PAMPs). Its receptor ectodomain recognizes the cleaved form of the cytokine spätzle, which is secreted in thehaemolymphas an inactive dimeric precursor. The toll receptor shares the cytoplasmatic TIR domain with mammalian TLRs, but the ectodomain and intracytoplasmatic tail are different. This difference might reflect a function of these receptors as cytokine receptors rather thanPRRs.
The toll pathway is activated by different stimuli, such asgram-positive bacteria,fungi, andvirulence factors.[17][20]First, the Spätzle processing enzyme (SPE) is activated in response to infection and cleavesspätzle(spz). Cleaved spätzle then binds to the toll receptor and crosslinks its ectodomains. This triggers conformational changes in the receptor resulting in signalling through toll. From this point forward, the signalling cascade is very similar to mammalian signalling through TLRs. The toll-induced signalling complex (TICS) is composed ofMyD88,Tube, and Pelle (the orthologue of mammalian IRAK). Signal from TICS is then transduced to Cactus (homologue of mammalianIκB), phosphorylated Cactus is polyubiquitylated and degraded, allowing nuclear translocation of DIF (dorsal-related immunity factor; a homologue of mammalianNF-κB) and induction of transcription of genes forantimicrobial peptides(AMPs) such asdrosomycin.[21]
Drosophilahave a total of 9tollfamily and 6spzfamily genes that interact with each other to differing degrees.[22]
TLR2[edit]
TLR2has also been designated as CD282 (cluster of differentiation 282).
TLR3[edit]
TLR3does not use the MyD88 dependent pathway. Its ligand is retroviral double-stranded RNA (dsRNA), which activates theTRIFdependent signalling pathway. To explore the role of this pathway in retroviral reprograming, knock down techniques of TLR3 or TRIF were prepared, and results showed that only the TLR3 pathway is required for full induction of target gene expression by the retrovirus expression vector. This retroviral expression of four transcriptional factors (Oct4,Sox2,Klf4andc-Myc;OSKM) inducespluripotencyin somatic cells. This is supported by study, which shows, that efficiency and amount of human iPSC generation, using retroviral vectors, is reduced by knockdown of the pathway with peptide inhibitors orshRNAknockdown of TLR3 or its adaptor protein TRIF. Taken together, stimulation of TLR3 causes great changes in chromatin remodeling and nuclear reprogramming, and activation of inflammatory pathways is required for these changes, induction of pluripotency genes and generation of human induced pluripotent stem cells (iPSC) colonies.[23]
TLR11[edit]
As noted above, human cells do not expressTLR11,but mice cells do. Mouse-specific TLR11 recognizes uropathogenicE.coliand the apicomplexan parasiteToxoplasma gondii.WithToxoplasmaits ligand is the protein profilin and the ligand forE. coliisflagellin.The flagellin from the enteropathogenSalmonellais also recognized by TLR11.[24]
As mouse TLR11 is able to recognizeSalmonellaeffectively, normal mice do not get infected by oralSalmonellaTyphi,which causes food- and waterborne gastroenteritis andtyphoid feverin humans. TLR11 deficientknockout mice,on the other hand, are efficiently infected. As a result, this knockout mouse can act as adisease modelof human typhoid fever.[25]
Summary of known mammalian TLRs[edit]
Toll-like receptors bind and become activated by different ligands, which, in turn, are located on different types of organisms or structures. They also have different adapters to respond to activation and are located sometimes at the cell surface and sometimes to internalcell compartments.Furthermore, they are expressed by different types ofleucocytesor othercell types:
| Receptor | Ligand(s)[26] | Ligand location[26] | Adapter(s) | Location | Cell types[26] |
|---|---|---|---|---|---|
| TLR 1 | multiple triacyllipopeptides | Bacterial lipoprotein | MyD88/MAL | cell surface |
|
| TLR 2 | multipleglycolipids | Bacterial peptidoglycans | MyD88/MAL | cell surface |
|
| multiple lipopeptides andproteolipids | Bacterial peptidoglycans | ||||
| lipoteichoic acid | Gram-positive bacteria | ||||
| HSP70 | Host cells | ||||
| zymosan(Beta-glucan) | Fungi | ||||
| Numerous others | |||||
| TLR 3 | double-stranded RNA,poly I:C | viruses | TRIF | cell compartment |
|
| TLR 4 | lipopolysaccharide | Gram-negative bacteria | MyD88/MAL/TRIF/TRAM | cell surface |
|
| severalheat shock proteins | Bacteria and host cells | ||||
| fibrinogen | host cells | ||||
| heparan sulfatefragments | host cells | ||||
| hyaluronic acidfragments | host cells | ||||
| nickel[31] | |||||
| Variousopioiddrugs | |||||
| TLR 5 | Bacterial flagellin | Bacteria | MyD88 | cell surface |
|
| Profilin[32] | Toxoplasma gondii | ||||
| TLR 6 | multiple diacyl lipopeptides | Mycoplasma | MyD88/MAL | cell surface |
|
| TLR 7 | imidazoquinoline | small synthetic compounds | MyD88 | cell compartment |
|
| loxoribine(aguanosineanalogue) | |||||
| bropirimine | |||||
| resiquimod | |||||
| single-stranded RNA | RNA viruses | ||||
| TLR 8 | small synthetic compounds; single-stranded Viral RNA, phagocytized bacterial RNA(24) | MyD88 | cell compartment |
| |
| TLR 9 | unmethylatedCpG OligodeoxynucleotideDNA | Bacteria, DNA viruses | MyD88 | cell compartment |
|
| TLR 10 | triacylated lipopeptides[34] | unknown | cell surface | ||
| TLR 11 | Profilin | Toxoplasma gondii[38] | MyD88 | cell compartment[39] |
|
| Flagellin | Bacteria (E. coli,Salmonella)[24] | ||||
| TLR 12 | Profilin | Toxoplasma gondii[40] | MyD88 | cell compartment |
|
| TLR 13[42][43] | bacterial ribosomal RNA sequence "CGGAAAGACC" (but not the methylated version)[44] | Virus, bacteria | MyD88, TAK-1 | cell compartment |
|
Ligands[edit]
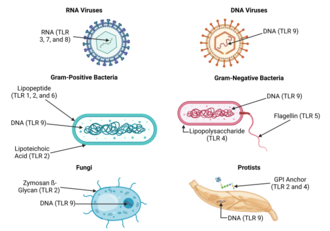
Because of the specificity of toll-like receptors (and other innate immune receptors) they cannot easily be changed in the course of evolution, these receptors recognize molecules that are constantly associated with threats (i.e., pathogen or cell stress) and are highly specific to these threats (i.e., cannot be mistaken for self molecules that are normally expressed under physiological conditions). Pathogen-associated molecules that meet this requirement are thought to be critical to the pathogen's function and difficult to change through mutation; they are said to be evolutionarily conserved. Somewhat conserved features in pathogens includebacterialcell-surfacelipopolysaccharides(LPS),lipoproteins,lipopeptides, andlipoarabinomannan;proteins such as flagellin from bacterialflagella;double-strandedRNAof viruses; or the unmethylatedCpGislands of bacterial and viralDNA;and also of the CpG islands found in the promoters of eukaryotic DNA; as well as certain other RNA and DNA molecules. As TLR ligands are present in most pathogens, they may also be present in pathogen-derived vaccines (e.g. MMR, influenza, polio vaccines) most commercially available vaccines have been assessed for their inherent TLR ligands' capacity to activate distinct subsets of immune cells.[45][46]For most of the TLRs,ligandrecognition specificity has now been established by gene targeting (also known as "gene knockout" ): a technique by which individual genes may be selectively deleted in mice.[47][48]See the table above for a summary of known TLR ligands.
Endogenous ligands[edit]
The stereotypic inflammatory response provoked by toll-like receptor activation has prompted speculation that endogenous activators of toll-like receptors might participate in autoimmune diseases. TLRs have been suspected of binding to host molecules includingfibrinogen(involved inblood clotting),heat shock proteins(HSPs),HMGB1,extracellular matrix components and self DNA (it is normally degraded by nucleases, but under inflammatory and autoimmune conditions it can form a complex with endogenous proteins, become resistant to these nucleases and gain access to endosomal TLRs as TLR7 or TLR9). These endogenous ligands are usually produced as a result of non-physiological cell death.[49]
Signaling[edit]
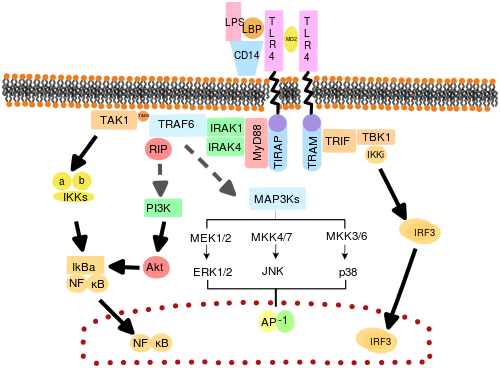
TLRs are believed to function asdimers.Though most TLRs appear to function ashomodimers,TLR2 formsheterodimerswith TLR1 or TLR6, each dimer having a different ligand specificity. TLRs may also depend on other co-receptors for full ligand sensitivity, such as in the case of TLR4's recognition ofLPS,which requires MD-2.CD14and LPS-Binding Protein (LBP) are known to facilitate the presentation of LPS to MD-2.
A set of endosomal TLRs comprising TLR3, TLR7, TLR8 and TLR9 recognizenucleic acidderived from viruses as well as endogenous nucleic acids in context of pathogenic events. Activation of these receptor leads to production of inflammatorycytokinesas well as type I interferons (interferon type I) to help fight viral infection.
The adapter proteins and kinases that mediate TLR signaling have also been targeted. In addition, random germline mutagenesis withENUhas been used to decipher the TLR signaling pathways. When activated, TLRs recruit adapter molecules within the cytoplasm of cells to propagate a signal. Four adapter molecules are known to be involved in signaling. These proteins are known asMyD88,TIRAP(also called Mal),TRIF,and TRAM (TRIF-related adaptor molecule).[50][51][52]
TLR signaling is divided into two distinct signaling pathways, the MyD88-dependent and TRIF-dependent pathway.
MyD88-dependent pathway[edit]
The MyD88-dependent response occurs on dimerization of TLRs, and is used by every TLR except TLR3. Its primary effect is activation of NFκB andMitogen-activated protein kinase.Ligand binding and conformational change that occurs in the receptor recruits the adaptor protein MyD88, a member of theTIRfamily. MyD88 then recruitsIRAK4,IRAK1andIRAK2.IRAK kinases then phosphorylate and activate the proteinTRAF6,which in turn polyubiquinates the protein TAK1, as well as itself to facilitate binding toIKK-β.On binding, TAK1 phosphorylates IKK-β, which then phosphorylates IκB causing its degradation and allowing NFκB to diffuse into the cell nucleus and activate transcription and consequent induction of inflammatory cytokines.[49]
TRIF-dependent pathway[edit]
Both TLR3 and TLR4 use the TRIF-dependent pathway, which is triggered bydsRNAand LPS, respectively. For TLR3, dsRNA leads to activation of the receptor, recruiting the adaptorTRIF.TRIF activates the kinasesTBK1andRIPK1,which creates a branch in the signaling pathway. The TRIF/TBK1 signaling complex phosphorylatesIRF3allowing its translocation into the nucleus and production ofInterferon type I.Meanwhile, activation of RIPK1 causes the polyubiquitination and activation of TAK1 and NFκB transcription in the same manner as the MyD88-dependent pathway.[49]
TLR signaling ultimately leads to the induction or suppression of genes that orchestrate the inflammatory response. In all, thousands of genes are activated by TLR signaling, and collectively, the TLRs constitute one of the mostpleiotropicyet tightly regulated gateways for gene modulation.
TLR4 is the only TLR that uses all four adaptors. Complex consisting of TLR4, MD2 and LPS recruits TIR domain-containing adaptors TIRAP and MyD88 and thus initiates activation of NFκB (early phase) and MAPK. TLR4-MD2-LPS complex then undergoes endocytosis and in endosome it forms a signalling complex with TRAM and TRIF adaptors. This TRIF-dependent pathway again leads to IRF3 activation and production of type I interferons, but it also activates late-phase NFκB activation. Both late and early phase activation of NFκB is required for production of inflammatory cytokines.[49]
Medical relevance[edit]
Imiquimod(cardinally used indermatology) is a TLR7 agonist, and its successorresiquimod,is a TLR7 and TLR8 agonist.[53]Recently, resiquimod has been explored as an agent for cancer immunotherapy,[54]acting through stimulation of tumor-associated macrophages.
Several TLR ligands are in clinical development or being tested in animal models asvaccine adjuvants,[55]with the first clinical use in humans in a recombinantherpes zoster vaccinein 2017, which contains a monophosphoryl lipid A component.
TLR7 messenger RNA expression levels in dairy animals in a natural outbreak of foot-and-mouth disease have been reported.[56]
TLR4has been shown to be important for the long-term side-effects ofopioids.Its activation leads to downstream release of inflammatory modulators includingTNF-αandIL-1β,and constant low-level release of these modulators is thought to reduce the efficacy of opioid drug treatment with time, and is involved in opioid tolerance,[57][58]hyperalgesiaandallodynia.[59][60]Morphine induced TLR4 activation attenuatespainsuppression byopioidsand enhances the development of opioidtoleranceandaddiction,drug abuse,and other negative side effects such asrespiratory depressionand hyperalgesia.[61]Drugs that block the action of TNF-α or IL-1β have been shown to increase the analgesic effects of opioids and reduce the development of tolerance and other side-effects,[62][63]and this has also been demonstrated with drugs that block TLR4 itself.
The "unnatural" enantiomers of opioid drugs such as (+)-morphine and (+)-naloxone lack affinity for opioid receptors, still produce the same activity at TLR4 as their "normal" enantiomers.[64][65]So, "unnatural" entianomers of opioids such as (+)-naloxone, can be used to block the TLR4 activity of opioid analgesic drugs without having any affinity for μ-opioid receptor[66][65][67]
Discovery[edit]
When microbes were first recognized as the cause of infectious diseases, it was immediately clear that multicellular organisms must be capable of recognizing them when infected and, hence, capable of recognizing molecules unique to microbes. A large body of literature, spanning most of the last century, attests to the search for the key molecules and their receptors. More than 100 years ago,Richard Pfeiffer,a student ofRobert Koch,coined the term "endotoxin"to describe a substance produced byGram-negative bacteriathat could provoke fever andshockinexperimental animals.In the decades that followed, endotoxin was chemically characterized and identified as alipopolysaccharide(LPS) produced by most Gram-negative bacteria. This lipopolysaccharide is an integral part of the gram-negative membrane and is released upon destruction of the bacterium. Other molecules (bacteriallipopeptides,flagellin,and unmethylatedDNA) were shown in turn to provoke host responses that are normally protective. However, these responses can be detrimental if they are excessively prolonged or intense. It followed logically that there must be receptors for such molecules, capable of alerting the host to the presence of infection, but these remained elusive for many years. Toll-like receptors are now counted among the key molecules that alert theimmune systemto the presence of microbial infections.
The prototypic member of the family, thetollreceptor (P08953;Tl) in the fruit flyDrosophila melanogaster,was discovered in 1985 by 1995 Nobel LaureatesChristiane Nüsslein-VolhardandEric Wieschausand colleagues. It was known for its developmental function inembryogenesisby establishing thedorsal-ventralaxis. It was named after Christiane Nüsslein-Volhard's 1985 exclamation, "Das ist jatoll!"(" That's amazing! "), in reference to the underdeveloped ventral portion of a fruit fly larva.[4]It wasclonedby the laboratory of Kathryn Anderson in 1988.[68]In 1996,tollwas found byJules A. Hoffmannand his colleagues to have an essential role in the fly's immunity tofungal infection,which it achieved by activating the synthesis of antimicrobial peptides.[19]
The first reported human toll-like receptor was described by Nomura and colleagues in 1994,[69]mapped to a chromosome by Taguchi and colleagues in 1996.[70]Because the immune function of toll inDrosophilawas not then known, it was assumed that TIL (now known as TLR1) might participate in mammalian development. However, in 1991 (prior to the discovery of TIL) it was observed that a molecule with a clear role in immune function in mammals, theinterleukin-1(IL-1) receptor, also had homology to drosophila toll; the cytoplasmic portions of both molecules were similar.[71]
In 1997,Charles JanewayandRuslan Medzhitovshowed that a toll-like receptor now known as TLR4 could, when artificially ligated using antibodies, induce the activation of certain genes necessary for initiating anadaptive immune response.[7]TLR 4 function as an LPS sensing receptor was discovered byBruce A. Beutlerand colleagues.[72]These workers usedpositional cloningto prove that mice that could not respond to LPS had mutations that abolished the function of TLR4. This identified TLR4 as one of the key components of the receptor for LPS.

In turn, the other TLR genes were ablated in mice by gene targeting, largely in the laboratory ofShizuo Akiraand colleagues. Each TLR is now believed to detect a discrete collection of molecules – some of microbial origin, and some products of cell damage – and to signal the presence of infections.[73]
Plant homologs oftollwere discovered by Pamela Ronald in 1995 (rice XA21)[74]and Thomas Boller in 2000 (ArabidopsisFLS2).[75]
In 2011, Beutler and Hoffmann were awarded the Nobel Prize in Medicine or Physiology for their work.[76]Hoffmann and Akira received the Canada Gairdner International Award in 2011.[77]
Notes and references[edit]
- ^Mahla RS, Reddy MC, Prasad DV, Kumar H (September 2013)."Sweeten PAMPs: Role of Sugar Complexed PAMPs in Innate Immunity and Vaccine Biology".Frontiers in Immunology.4:248.doi:10.3389/fimmu.2013.00248.PMC3759294.PMID24032031.
- ^Fore, Faith; Indriputri, Cut; Mamutse, Janet; Nugraha, Jusak (2020)."TLR10 and Its Unique Anti-Inflammatory Properties and Potential Use as a Target in Therapeutics".Immune Network.20(3): e21.doi:10.4110/in.2020.20.e21.ISSN1598-2629.PMC7327153.PMID32655969.
- ^Kemball CC, Alirezaei M, Whitton JL (2010)."Type B coxsackieviruses and their interactions with the innate and adaptive immune systems".Future Microbiology.5(9): 1329–1347.doi:10.2217/fmb.10.101.PMC3045535.PMID20860480.
- ^abHansson GK, Edfeldt K (June 2005)."Toll to be paid at the gateway to the vessel wall".Arteriosclerosis, Thrombosis, and Vascular Biology.25(6): 1085–7.doi:10.1161/01.ATV.0000168894.43759.47.PMID15923538.
- ^Delneste Y, Beauvillain C, Jeannin P (January 2007)."[Innate immunity: structure and function of TLRs]".Médecine/Sciences.23(1): 67–73.doi:10.1051/medsci/200723167.PMID17212934.
- ^Takeda K, Akira S (January 2005). "Toll-like receptors in innate immunity".International Immunology.17(1): 1–14.doi:10.1093/intimm/dxh186.PMID15585605.
- ^abcMedzhitov R, Preston-Hurlburt P, Janeway CA (July 1997)."A human homologue of the Drosophila Toll protein signals activation of adaptive immunity".Nature.388(6640): 394–7.Bibcode:1997Natur.388..394M.doi:10.1038/41131.PMID9237759.
- ^abSharma N, Akhade AS, Qadri A (April 2013). "Sphingosine-1-phosphate suppresses TLR-induced CXCL8 secretion from human T cells".Journal of Leukocyte Biology.93(4): 521–8.doi:10.1189/jlb.0712328.PMID23345392.
- ^Sameer AS, Nissar S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. Biomed Res Int. 2021 Sep 12;2021:1157023. doi: 10.1155/2021/1157023. PMID 34552981; PMCID: PMC8452412.
- ^Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M (September 2007). "Toll-like receptors modulate adult hippocampal neurogenesis".Nature Cell Biology.9(9): 1081–8.doi:10.1038/ncb1629.PMID17704767.S2CID12517461.
- ^Du X, Poltorak A, Wei Y, Beutler B (September 2000)."Three novel mammalian toll-like receptors: gene structure, expression, and evolution".European Cytokine Network.11(3): 362–71.PMID11022119.
- ^Chuang TH, Ulevitch RJ (September 2000)."Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9".European Cytokine Network.11(3): 372–8.PMID11022120.
- ^Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, et al. (March 2004)."Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection".Proceedings of the National Academy of Sciences of the United States of America.101(10): 3516–21.Bibcode:2004PNAS..101.3516T.doi:10.1073/pnas.0400525101.PMC373494.PMID14993594.
- ^abRoach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, et al. (July 2005)."The evolution of vertebrate Toll-like receptors".Proceedings of the National Academy of Sciences of the United States of America.102(27): 9577–82.Bibcode:2005PNAS..102.9577R.doi:10.1073/pnas.0502272102.PMC1172252.PMID15976025.
- ^Lemaitre B, Hoffmann J (2007)."The host defense of Drosophila melanogaster".Annual Review of Immunology.25:697–743.doi:10.1146/annurev.immunol.25.022106.141615.PMID17201680.
- ^Valanne S, Wang JH, Rämet M (January 2011)."The Drosophila Toll signaling pathway".Journal of Immunology.186(2): 649–56.doi:10.4049/jimmunol.1002302.PMID21209287.
- ^abDudzic JP, Hanson MA, Iatsenko I, Kondo S, Lemaitre B (April 2019)."More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila".Cell Reports.27(4): 1050–1061.e3.doi:10.1016/j.celrep.2019.03.101.PMID31018123.
- ^Hanson MA, Hamilton PT, Perlman SJ (October 2016)."Immune genes and divergent antimicrobial peptides in flies of the subgenus Drosophila".BMC Evolutionary Biology.16(1): 228.doi:10.1186/s12862-016-0805-y.PMC5078906.PMID27776480.
- ^abLemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (September 1996)."The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults".Cell.86(6): 973–83.doi:10.1016/s0092-8674(00)80172-5.PMID8808632.S2CID10736743.
- ^Issa N, Guillaumot N, Lauret E, Matt N, Schaeffer-Reiss C, Van Dorsselaer A, et al. (February 2018)."The Circulating Protease Persephone Is an Immune Sensor for Microbial Proteolytic Activities Upstream of the Drosophila Toll Pathway".Molecular Cell.69(4): 539–550.e6.doi:10.1016/j.molcel.2018.01.029.PMC5823974.PMID29452635.
- ^Ferrandon D, Imler JL, Hetru C, Hoffmann JA (November 2007). "The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections".Nature Reviews. Immunology.7(11): 862–74.doi:10.1038/nri2194.PMID17948019.S2CID11104900.
- ^Chowdhury M, Li CF, He Z, Lu Y, Liu XS, Wang YF, et al. (June 2019)."Drosophila".The Journal of Biological Chemistry.294(26): 10172–10181.doi:10.1074/jbc.RA118.006804.PMC6664172.PMID31088910.
- ^Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, et al. (October 2012)."Activation of innate immunity is required for efficient nuclear reprogramming".Cell.151(3): 547–58.doi:10.1016/j.cell.2012.09.034.PMC3506423.PMID23101625.
- ^abHatai, Hirotsugu; Lepelley, Alice; Zeng, Wangyong; Hayden, Matthew S.; Ghosh, Sankar (2016)."Toll-Like Receptor 11 (TLR11) Interacts with Flagellin and Profilin through Disparate Mechanisms".PLOS ONE.11(2): e0148987.Bibcode:2016PLoSO..1148987H.doi:10.1371/journal.pone.0148987.ISSN1932-6203.PMC4747465.PMID26859749.
- ^Mathur R, Oh H, Zhang D, Park SG, Seo J, Koblansky A, et al. (October 2012)."A mouse model of Salmonella typhi infection".Cell.151(3): 590–602.doi:10.1016/j.cell.2012.08.042.PMC3500584.PMID23101627.
- ^abcUnless else specified in boxes then ref is:Waltenbaugh C, Doan T, Melvold R, Viselli S (2008).Immunology.Lippincott's Illustrated reviews. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 17.ISBN978-0-7817-9543-2.
- ^abSabroe I, Dower SK, Whyte MK (November 2005)."The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis".Clinical Infectious Diseases.41(Suppl 7): S421-6.doi:10.1086/431992.PMID16237641.
- ^abcdSallusto F, Lanzavecchia A (2002)."The instructive role of dendritic cells on T-cell responses".Arthritis Research.4(Suppl 3): S127-32.doi:10.1186/ar567.PMC3240143.PMID12110131.
- ^Gerondakis S, Grumont RJ, Banerjee A (2007)."Regulating B-cell activation and survival in response to TLR signals".Immunology and Cell Biology.85(6): 471–5.doi:10.1038/sj.icb.7100097.PMID17637697.S2CID30443009.
- ^Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK (January 2000)."Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors".Journal of Immunology.164(2): 966–72.doi:10.4049/jimmunol.164.2.966.PMID10623846.
- ^Peana M, Zdyb K, Medici S, Pelucelli A, Simula G, Gumienna-Kontecka E, Zoroddu MA (December 2017). "Ni(II) interaction with a peptide model of the human TLR4 ectodomain".Journal of Trace Elements in Medicine and Biology.44:151–160.doi:10.1016/j.jtemb.2017.07.006.PMID28965571.
- ^Salazar Gonzalez RM, Shehata H, O'Connell MJ, Yang Y, Moreno-Fernandez ME, Chougnet CA, Aliberti J (2014)."Toxoplasma gondii- derived profilin triggers human toll-like receptor 5-dependent cytokine production".Journal of Innate Immunity.6(5): 685–94.doi:10.1159/000362367.PMC4141014.PMID24861338.
- ^Seizer L, Rahimi S, Santos-Sierra S, Drexel M (2022) Expression of toll like receptor 8 (TLR8) in specific groups of mouse hippocampal interneurons. PLoS ONE 17(5): e0267860.https://doi.org/10.1371/journal.pone.0267860
- ^Guan Y, Ranoa DR, Jiang S, Mutha SK, Li X, Baudry J, Tapping RI (May 2010)."Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling".Journal of Immunology.184(9): 5094–103.doi:10.4049/jimmunol.0901888.PMID20348427.
- ^Chuang T, Ulevitch RJ (March 2001). "Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells".Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression.1518(1–2): 157–61.doi:10.1016/s0167-4781(00)00289-x.PMID11267672.
- ^Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S, Hartmann G (May 2002)."Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides".Journal of Immunology.168(9). Baltimore, Md.: 1950: 4531–7.doi:10.4049/jimmunol.168.9.4531.PMID11970999.
{{cite journal}}:CS1 maint: location (link) - ^abRegan T, Nally K, Carmody R, Houston A, Shanahan F, Macsharry J, Brint E (December 2013)."Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages".Journal of Immunology.191(12): 6084–92.doi:10.4049/jimmunol.1203245.PMID24198280.
- ^Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, et al. (June 2005)."TLR11 activation of dendritic cells by a protozoan profilin-like protein".Science.308(5728): 1626–9.Bibcode:2005Sci...308.1626Y.doi:10.1126/science.1109893.PMID15860593.S2CID34165967.
- ^Pifer R, Benson A, Sturge CR, Yarovinsky F (February 2011)."UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii".The Journal of Biological Chemistry.286(5): 3307–14.doi:10.1074/jbc.M110.171025.PMC3030336.PMID21097503.
- ^Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, et al. (January 2013)."Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii".Immunity.38(1): 119–30.doi:10.1016/j.immuni.2012.09.016.PMC3601573.PMID23246311.
- ^Mishra BB, Gundra UM, Teale JM (December 2008)."Expression and distribution of Toll-like receptors 11-13 in the brain during murine neurocysticercosis".Journal of Neuroinflammation.5:53.doi:10.1186/1742-2094-5-53.PMC2631477.PMID19077284.
- ^Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, et al. (February 2011)."A novel Toll-like receptor that recognizes vesicular stomatitis virus".The Journal of Biological Chemistry.286(6): 4517–24.doi:10.1074/jbc.M110.159590.PMC3039399.PMID21131352.
- ^Oldenburg M, Krüger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, et al. (August 2012). "TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification".Science.337(6098): 1111–5.Bibcode:2012Sci...337.1111O.doi:10.1126/science.1220363.PMID22821982.S2CID206540638.
- ^Hochrein H, Kirschning CJ (March 2013)."Bacteria evade immune recognition via TLR13 and binding of their 23S rRNA by MLS antibiotics by the same mechanisms".Oncoimmunology.2(3): e23141.doi:10.4161/onci.23141.PMC3661153.PMID23802068.
- ^Schreibelt, Gerty; Benitez-Ribas, Daniel; Schuurhuis, Danita; Lambeck, Annechien J. A.; van Hout-Kuijer, Maaike; Schaft, Niels; Punt, Cornelis J. A.; Figdor, Carl G.; Adema, Gosse J.; de Vries, I. Jolanda M. (29 July 2010)."Commonly used prophylactic vaccines as an alternative for synthetically produced TLR ligands to mature monocyte-derived dendritic cells".Blood.116(4): 564–574.doi:10.1182/blood-2009-11-251884.hdl:2066/89493.ISSN1528-0020.PMID20424184.
- ^Aleynick, Mark; Svensson-Arvelund, Judit; Pantsulaia, Gvantsa; Kim, Kristy; Rose, Samuel A.; Upadhyay, Ranjan; Yellin, Michael; Marsh, Henry; Oreper, Daniel; Jhunjhunwala, Suchit; Moussion, Christine Carine; Merad, Miriam; Brown, Brian D.; Brody, Joshua D. (July 2023)."Pattern recognition receptor agonists in pathogen vaccines mediate antitumor T-cell cross-priming".Journal for Immunotherapy of Cancer.11(7): e007198.doi:10.1136/jitc-2023-007198.ISSN2051-1426.PMC10373699.PMID37487664.
- ^Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, et al. (August 2003). "Identification of Lps2 as a key transducer of MyD88-independent TIR signalling".Nature.424(6950): 743–8.Bibcode:2003Natur.424..743H.doi:10.1038/nature01889.PMID12872135.S2CID15608748.
- ^Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. (December 2000). "A Toll-like receptor recognizes bacterial DNA".Nature.408(6813): 740–5.Bibcode:2000Natur.408..740H.doi:10.1038/35047123.PMID11130078.S2CID4405163.
- ^abcdKawai T, Akira S (May 2010)."The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors".Nature Immunology.11(5): 373–84.doi:10.1038/ni.1863.PMID20404851.S2CID39414949.
- ^Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, et al. (May 2007)."TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways".Journal of Immunology.178(10): 6252–8.doi:10.4049/jimmunol.178.10.6252.PMID17475853.
- ^Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, et al. (November 2003). "TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway".Nature Immunology.4(11): 1144–50.doi:10.1038/ni986.PMID14556004.S2CID13016860.
- ^Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, et al. (November 2002). "Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4".Nature.420(6913): 324–9.Bibcode:2002Natur.420..324Y.doi:10.1038/nature01182.PMID12447441.S2CID16163262.
- ^Peter Fritsch (2004).Dermatologie Venerologie: Grundlagen. Klinik. Atlas(in German). Berlin: Springer.ISBN3-540-00332-0.
- ^Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. (August 2018)."TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy".Nature Biomedical Engineering.2(8): 578–588.doi:10.1038/s41551-018-0236-8.PMC6192054.PMID31015631.
- ^Toussi DN, Massari P (April 2014)."Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands".Vaccines.2(2): 323–53.doi:10.3390/vaccines2020323.PMC4494261.PMID26344622.
- ^Audarya, S.D.; Pattnaik, B.; Sanyal, A.; Mohapatra, J.K. (2017)."Toll like Receptor 7 Messenger Ribonucleic Acid Expression Levels in Dairy Animals in an Outbreak of Foot-and-mouth disease"(PDF).Buffalo Bulletin.36(3). Archived fromthe original(PDF)on 28 April 2021.
- ^Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R (May 2005). "Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance".Pain.115(1–2): 50–9.doi:10.1016/j.pain.2005.02.003.PMID15836969.S2CID7286123.
- ^Mohan S, Davis RL, DeSilva U, Stevens CW (October 2010)."Dual regulation of mu opioid receptors in SK-N-SH neuroblastoma cells by morphine and interleukin-1β: evidence for opioid-immune crosstalk".Journal of Neuroimmunology.227(1–2): 26–34.doi:10.1016/j.jneuroim.2010.06.007.PMC2942958.PMID20615556.
- ^Komatsu T, Sakurada S, Katsuyama S, Sanai K, Sakurada T (2009).Mechanism of allodynia evoked by intrathecal morphine-3-glucuronide in mice.International Review of Neurobiology. Vol. 85. pp. 207–19.doi:10.1016/S0074-7742(09)85016-2.ISBN9780123748935.PMID19607972.
- ^Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR (January 2010)."Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta".Neuroscience.165(2): 569–83.doi:10.1016/j.neuroscience.2009.10.011.PMC2795035.PMID19833175.
- ^Drahl C (22 August 2012)."Small Molecules Target Toll-Like Receptors".Chemical & Engineering News.
- ^Shen CH, Tsai RY, Shih MS, Lin SL, Tai YH, Chien CC, Wong CS (February 2011)."Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats".Anesthesia and Analgesia.112(2): 454–9.doi:10.1213/ANE.0b013e3182025b15.PMID21081778.S2CID12295407.
- ^Hook MA, Washburn SN, Moreno G, Woller SA, Puga D, Lee KH, Grau JW (February 2011)."An IL-1 receptor antagonist blocks a morphine-induced attenuation of locomotor recovery after spinal cord injury".Brain, Behavior, and Immunity.25(2): 349–59.doi:10.1016/j.bbi.2010.10.018.PMC3025088.PMID20974246.
- ^Watkins LR, Hutchinson MR, Rice KC, Maier SF (November 2009)."The" toll "of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia".Trends in Pharmacological Sciences.30(11): 581–91.doi:10.1016/j.tips.2009.08.002.PMC2783351.PMID19762094.
- ^abHutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR (July 2008)."Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4)".The European Journal of Neuroscience.28(1): 20–9.doi:10.1111/j.1460-9568.2008.06321.x.PMC2588470.PMID18662331.
- ^Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR (November 2008)."Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia".Brain, Behavior, and Immunity.22(8): 1178–89.doi:10.1016/j.bbi.2008.05.004.PMC2783238.PMID18599265.
- ^Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR (May 2010)."Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences".Neuroscience.167(3): 880–93.doi:10.1016/j.neuroscience.2010.02.011.PMC2854318.PMID20178837.
- ^Hashimoto C, Hudson KL, Anderson KV (January 1988). "The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein".Cell.52(2): 269–79.doi:10.1016/0092-8674(88)90516-8.PMID2449285.S2CID19439405.
- ^Nomura N, Miyajima N, Sazuka T, Tanaka A, Kawarabayasi Y, Sato S, et al. (1994)."Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1".DNA Research.1(1): 27–35.doi:10.1093/dnares/1.1.27.PMID7584026.
- ^Taguchi T, Mitcham JL, Dower SK, Sims JE, Testa JR (March 1996). "Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor Toll, to human chromosome 4p14".Genomics.32(3): 486–8.doi:10.1006/geno.1996.0150.PMID8838819.
- ^Gay NJ, Keith FJ (May 1991). "Drosophila Toll and IL-1 receptor".Nature.351(6325): 355–6.Bibcode:1991Natur.351..355G.doi:10.1038/351355b0.PMID1851964.S2CID1700458.
- ^Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. (December 1998). "Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene".Science.282(5396): 2085–8.Bibcode:1998Sci...282.2085P.doi:10.1126/science.282.5396.2085.PMID9851930.
- ^Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. (April 1999)."Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product".Journal of Immunology.162(7): 3749–52.doi:10.4049/jimmunol.162.7.3749.PMID10201887.S2CID7419784.
- ^Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, et al. (December 1995)."A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21".Science.270(5243): 1804–6.Bibcode:1995Sci...270.1804S.doi:10.1126/science.270.5243.1804.PMID8525370.S2CID10548988.
- ^Gómez-Gómez L, Boller T (June 2000)."FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis".Molecular Cell.5(6): 1003–11.doi:10.1016/S1097-2765(00)80265-8.PMID10911994.
- ^"The Nobel Prize in Physiology or Medicine 2011".Nobel Media AB.3 October 2011.
- ^Mitchell B (23 March 2011)."B.C. doctor wins prestigious medical prize".The Star.
See also[edit]
External links[edit]
- Toll-Like+Receptorsat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- Toll+protein,+Drosophilaat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- TollML: Toll-like receptors and ligands databaseatUniversity of Munich
- The Toll-Like Receptor Family of Innate Immune Receptors (pdf)
- Toll-Like receptor Pathway
- BioScience Animations
