Transferase
Inbiochemistry,atransferaseis any one of a class ofenzymesthat catalyse the transfer of specificfunctional groups(e.g. amethylorglycosylgroup) from onemolecule(called the donor) to another (called the acceptor).[2]They are involved in hundreds of differentbiochemical pathwaysthroughout biology, and are integral to some of life's most important processes.
Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity ofcoenzyme A(CoA)transferase,which transfersthiol esters,[3]the action ofN-acetyltransferase,which is part of the pathway that metabolizestryptophan,[4]and the regulation ofpyruvate dehydrogenase(PDH), which convertspyruvatetoacetyl CoA.[5]Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by apeptidyl transferase.The transfer involves the removal of the growingamino acidchain from thetRNAmolecule in theA-siteof theribosomeand its subsequent addition to the amino acid attached to the tRNA in theP-site.[6]
Mechanistically, an enzyme that catalyzed the following reaction would be a transferase:
In the above reaction (where the dash represents a bond, not a minus sign), X would be the donor, and Y would be the acceptor.[7]R denotes the functional group transferred as a result of transferase activity. The donor is often acoenzyme.
History
[edit]Some of the most important discoveries relating to transferases occurred as early as the 1930s. Earliest discoveries of transferase activity occurred in other classifications ofenzymes,includingbeta-galactosidase,protease,and acid/basephosphatase.Prior to the realization that individual enzymes were capable of such a task, it was believed that two or more enzymes enacted functional group transfers.[8]

Transamination,or the transfer of anamine(or NH2) group from an amino acid to aketo acidby anaminotransferase(also known as a "transaminase" ), was first noted in 1930 byDorothy M. Needham,after observing the disappearance ofglutamic acidadded to pigeon breast muscle.[9]This observance was later verified by the discovery of its reaction mechanism by Braunstein and Kritzmann in 1937.[10]Their analysis showed that this reversible reaction could be applied to other tissues.[11]This assertion was validated byRudolf Schoenheimer's work withradioisotopesastracersin 1937.[12][13]This in turn would pave the way for the possibility that similar transfers were a primary means of producing most amino acids via amino transfer.[14]
Another such example of early transferase research and later reclassification involved the discovery of uridyl transferase. In 1953, the enzymeUDP-glucose pyrophosphorylasewas shown to be a transferase, when it was found that it could reversibly produceUTPandG1PfromUDP-glucoseand an organicpyrophosphate.[15]
Another example of historical significance relating to transferase is the discovery of the mechanism ofcatecholaminebreakdown bycatechol-O-methyltransferase.This discovery was a large part of the reason forJulius Axelrod’s 1970Nobel Prize in Physiology or Medicine(shared withSir Bernard KatzandUlf von Euler).[16]
Classification of transferases continues to this day, with new ones being discovered frequently.[17][18]An example of this is Pipe, a sulfotransferase involved in the dorsal-ventral patterning ofDrosophila.[19]Initially, the exact mechanism of Pipe was unknown, due to a lack of information on its substrate.[20]Research into Pipe's catalytic activity eliminated the likelihood of it being a heparan sulfateglycosaminoglycan.[21]Further research has shown that Pipe targets the ovarian structures for sulfation.[22]Pipe is currently classified as aDrosophilaheparan sulfate 2-O-sulfotransferase.[23]
Nomenclature
[edit]Systematic namesof transferases are constructed in the form of "donor:acceptor grouptransferase."[24]For example, methylamine:L-glutamate N-methyltransferase would be the standard naming convention for the transferasemethylamine-glutamate N-methyltransferase,wheremethylamineis the donor,L-glutamateis the acceptor, andmethyltransferaseis the EC category grouping. This same action by the transferase can be illustrated as follows:
- methylamine + L-glutamateNH3+N-methyl-L-glutamate[25]
However, other accepted names are more frequently used for transferases, and are often formed as "acceptor grouptransferase" or "donor grouptransferase." For example, aDNA methyltransferaseis a transferase that catalyzes the transfer of amethylgroup to aDNAacceptor. In practice, many molecules are not referred to using this terminology due to more prevalent common names.[26]For example,RNA polymeraseis the modern common name for what was formerly known as RNA nucleotidyltransferase, a kind ofnucleotidyl transferasethat transfersnucleotidesto the 3’ end of a growingRNAstrand.[27]In the EC system of classification, the accepted name for RNA polymerase is DNA-directed RNA polymerase.[28]
Classification
[edit]Described primarily based on the type of biochemical group transferred, transferases can be divided into ten categories (based on theEC Numberclassification).[29]These categories comprise over 450 different unique enzymes.[30]In the EC numbering system, transferases have been given a classification ofEC2.Hydrogenis not considered a functional group when it comes to transferase targets; instead, hydrogen transfer is included underoxidoreductases,[30]due to electron transfer considerations.
| EC number | Examples | Group(s) transferred |
|---|---|---|
| EC 2.1 | methyltransferaseandformyltransferase | single-carbongroups |
| EC 2.2 | transketolaseandtransaldolase | aldehydeorketonegroups |
| EC 2.3 | acyltransferase | acylgroups or groups that becomealkylgroups during transfer |
| EC 2.4 | glycosyltransferase,hexosyltransferase,andpentosyltransferase | glycosylgroups, as well ashexosesandpentoses |
| EC 2.5 | riboflavin synthaseandchlorophyll synthase | alkylorarylgroups, other than methyl groups |
| EC 2.6 | transaminase,andoximinotransferase | nitrogenousgroups |
| EC 2.7 | phosphotransferase,polymerase,andkinase | phosphorus-containing groups; subclasses are based on the acceptor (e.g.alcohol,carboxyl,etc.) |
| EC 2.8 | sulfurtransferaseandsulfotransferase | sulfur-containing groups |
| EC 2.9 | selenotransferase | selenium-containing groups |
| EC 2.10 | molybdenumtransferaseandtungstentransferase | molybdenumortungsten |
Role
[edit]EC 2.1: single carbon transferases
[edit]
EC 2.1 includes enzymes that transfer single-carbon groups. This category consists of transfers ofmethyl,hydroxymethyl,formyl, carboxy,carbamoyl,and amido groups.[31]Carbamoyltransferases, as an example, transfer a carbamoyl group from one molecule to another.[32]Carbamoyl groups follow the formula NH2CO.[33]InATCasesuch a transfer is written ascarbamoyl phosphate+ L-aspartateL-carbamoyl aspartate +phosphate.[34]
EC 2.2: aldehyde and ketone transferases
[edit]
Enzymes that transfer aldehyde or ketone groups and included in EC 2.2. This category consists of various transketolases and transaldolases.[35]Transaldolase, the namesake of aldehyde transferases, is an important part of the pentose phosphate pathway.[36]The reaction it catalyzes consists of a transfer of a dihydroxyacetone functional group toglyceraldehyde 3-phosphate(also known as G3P). The reaction is as follows:sedoheptulose 7-phosphate+ glyceraldehyde 3-phosphateerythrose 4-phosphate+fructose 6-phosphate.[37]
EC 2.3: acyl transferases
[edit]Transfer of acyl groups or acyl groups that become alkyl groups during the process of being transferred are key aspects of EC 2.3. Further, this category also differentiates between amino-acyl and non-amino-acyl groups.Peptidyl transferaseis aribozymethat facilitates formation ofpeptide bondsduringtranslation.[38]As an aminoacyltransferase, it catalyzes the transfer of a peptide to anaminoacyl-tRNA,following this reaction: peptidyl-tRNAA+ aminoacyl-tRNABtRNAA+ peptidyl aminoacyl-tRNAB.[39]
EC 2.4: glycosyl, hexosyl, and pentosyl transferases
[edit]EC 2.4 includes enzymes that transferglycosylgroups, as well as those that transfer hexose and pentose.Glycosyltransferaseis a subcategory of EC 2.4 transferases that is involved inbiosynthesisofdisaccharidesandpolysaccharidesthrough transfer ofmonosaccharidesto other molecules.[40]An example of a prominent glycosyltransferase islactose synthasewhich is a dimer possessing twoprotein subunits.Its primary action is to producelactosefromglucoseand UDP-galactose.[41]This occurs via the following pathway: UDP-β-D-galactose + D-glucoseUDP+ lactose.[42]
EC 2.5: alkyl and aryl transferases
[edit]EC 2.5 relates to enzymes that transfer alkyl or aryl groups, but does not include methyl groups. This is in contrast to functional groups that become alkyl groups when transferred, as those are included in EC 2.3. EC 2.5 currently only possesses one sub-class: Alkyl and aryl transferases.[43]Cysteine synthase,for example, catalyzes the formation of acetic acids andcysteinefrom O3-acetyl-L-serine and hydrogen sulfide: O3-acetyl-L-serine + H2SL-cysteine + acetate.[44]
EC 2.6: nitrogenous transferases
[edit]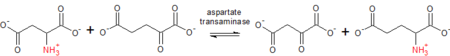
The grouping consistent with transfer ofnitrogenousgroups is EC 2.6. This includes enzymes liketransaminase(also known as "aminotransferase" ), and a very small number ofoximinotransferasesand other nitrogen group transferring enzymes. EC 2.6 previously includedamidinotransferasebut it has since been reclassified as a subcategory of EC 2.1 (single-carbon transferring enzymes).[45]In the case ofaspartate transaminase,which can act ontyrosine,phenylalanine,andtryptophan,it reversibly transfers anaminogroup from one molecule to the other.[46]
The reaction, for example, follows the following order: L-aspartate +2-oxoglutarateoxaloacetate + L-glutamate.[47]
EC 2.7: phosphorus transferases
[edit]While EC 2.7 includes enzymes that transferphosphorus-containing groups, it also includes nuclotidyl transferases as well.[48]Sub-categoryphosphotransferaseis divided up in categories based on the type of group that accepts the transfer.[24]Groups that are classified as phosphate acceptors include: alcohols, carboxy groups, nitrogenous groups, and phosphate groups.[29]Further constituents of this subclass of transferases are various kinases. A prominent kinase iscyclin-dependent kinase(or CDK), which comprises a sub-family ofprotein kinases.As their name implies, CDKs are heavily dependent on specificcyclinmolecules foractivation.[49]Once combined, the CDK-cyclin complex is capable of enacting its function within the cell cycle.[50]
The reaction catalyzed by CDK is as follows: ATP + a target proteinADP + a phosphoprotein.[51]
EC 2.8: sulfur transferases
[edit]
Transfer of sulfur-containing groups is covered by EC 2.8 and is subdivided into the subcategories of sulfurtransferases, sulfotransferases, and CoA-transferases, as well as enzymes that transfer alkylthio groups.[53]A specific group of sulfotransferases are those that usePAPSas a sulfate group donor.[54]Within this group isalcohol sulfotransferasewhich has a broad targeting capacity.[55]Due to this, alcohol sulfotransferase is also known by several other names including "hydroxysteroid sulfotransferase," "steroid sulfokinase," and "estrogen sulfotransferase."[56]Decreases in its activity has been linked to human liver disease.[57]This transferase acts via the following reaction: 3'-phosphoadenylyl sulfate + an alcoholadenosine 3',5'bisphosphate + an alkyl sulfate.[58]
EC 2.9: selenium transferases
[edit]EC 2.9 includes enzymes that transferselenium-containing groups.[59]This category only contains two transferases, and thus is one of the smallest categories of transferase. Selenocysteine synthase, which was first added to the classification system in 1999, converts seryl-tRNA(Sec UCA) into selenocysteyl-tRNA(Sec UCA).[60]
EC 2.10: metal transferases
[edit]The category of EC 2.10 includes enzymes that transfermolybdenumortungsten-containing groups. However, as of 2011, only one enzyme has been added:molybdopterin molybdotransferase.[61]This enzyme is a component of MoCo biosynthesis inEscherichia coli.[62]The reaction it catalyzes is as follows: adenylyl-molybdopterin+molybdatemolybdenum cofactor + AMP.[63]
Role in histo-blood group
[edit]The A and B transferases are the foundation of the humanABO blood groupsystem. Both A and B transferases are glycosyltransferases, meaning they transfer a sugar molecule onto an H-antigen.[64]This allows H-antigen to synthesize theglycoproteinandglycolipidconjugates that are known as the A/Bantigens.[64]The full name of A transferase is alpha 1-3-N-acetylgalactosaminyltransferase[65]and its function in the cell is to add N-acetylgalactosamine to H-antigen, creating A-antigen.[66]: 55 The full name of B transferase is alpha 1-3-galactosyltransferase,[65]and its function in the cell is to add agalactosemolecule to H-antigen, creating B-antigen.[66]
It is possible forHomo sapiensto have any of four differentblood types:Type A (express A antigens), Type B (express B antigens), Type AB (express both A and B antigens) and Type O (express neither A nor B antigens).[67]The gene for A and B transferases is located onchromosome 9.[68]The gene contains sevenexonsand sixintrons[69]and the gene itself is over 18kb long.[70]The alleles for A and B transferases are extremely similar. The resulting enzymes only differ in 4 amino acid residues.[66]The differing residues are located at positions 176, 235, 266, and 268 in the enzymes.[66]: 82–83
Deficiencies
[edit]
.
Transferasedeficienciesare at the root of many commonillnesses.The most common result of a transferase deficiency is a buildup of acellular product.
SCOT deficiency
[edit]Succinyl-CoA:3-ketoacid CoAtransferase deficiency (orSCOT deficiency) leads to a buildup ofketones.[71] Ketonesare created upon the breakdown of fats in the body and are an important energy source.[72]Inability to utilizeketonesleads to intermittentketoacidosis,which usually first manifests during infancy.[72]Disease sufferers experience nausea, vomiting, inability to feed, and breathing difficulties.[72]In extreme cases, ketoacidosis can lead to coma and death.[72]The deficiency is caused bymutationin the gene OXCT1.[73]Treatments mostly rely on controlling the diet of the patient.[74]
CPT-II deficiency
[edit]Carnitine palmitoyltransferase IIdeficiency (also known asCPT-II deficiency) leads to an excess long chainfatty acids,as thebodylacks the ability to transport fatty acids into themitochondriato be processed as a fuel source.[75]The disease is caused by a defect in the gene CPT2.[76]This deficiency will present in patients in one of three ways: lethal neonatal, severe infantile hepatocardiomuscular, and myopathic form.[76]The myopathic is the least severe form of the deficiency and can manifest at any point in the lifespan of the patient.[76]The other two forms appear in infancy.[76]Common symptoms of the lethal neonatal form and the severe infantile forms are liver failure, heart problems, seizures and death.[76]The myopathic form is characterized by muscle pain and weakness following vigorous exercise.[76]Treatment generally includes dietary modifications and carnitine supplements.[76]
Galactosemia
[edit]Galactosemiaresults from an inability to process galactose, asimple sugar.[77]This deficiency occurs when the gene forgalactose-1-phosphate uridylyltransferase(GALT) has any number of mutations, leading to a deficiency in the amount of GALT produced.[78][79]There are two forms of Galactosemia: classic and Duarte.[80]Duarte galactosemiais generally less severe than classic galactosemia and is caused by a deficiency ofgalactokinase.[81]Galactosemia renders infants unable to process the sugars in breast milk, which leads to vomiting andanorexiawithin days of birth.[81]Most symptoms of the disease are caused by a buildup ofgalactose-1-phosphatein the body.[81]Common symptoms include liver failure,sepsis,failure to grow, and mental impairment, among others.[82]Buildup of a second toxic substance,galactitol,occurs in the lenses of the eyes, causingcataracts.[83]Currently, the only available treatment is early diagnosis followed by adherence to a diet devoid of lactose, and prescription of antibiotics for infections that may develop.[84]
Choline acetyltransferase deficiencies
[edit]Choline acetyltransferase(also known as ChAT or CAT) is an important enzyme which produces theneurotransmitteracetylcholine.[85]Acetylcholine is involved in many neuropsychic functions such as memory, attention, sleep and arousal.[86][87][88] The enzyme is globular in shape and consists of a single amino acid chain.[89]ChAT functions to transfer anacetyl groupfrom acetyl co-enzyme A tocholinein thesynapsesofnervecells and exists in two forms: soluble and membrane bound.[89]The ChAT gene is located onchromosome 10.[90]
Alzheimer's disease
[edit]Decreased expression of ChAT is one of the hallmarks ofAlzheimer's disease.[91]Patients with Alzheimer's disease show a 30 to 90% reduction in activity in several regions of the brain, including thetemporal lobe,theparietal lobeand thefrontal lobe.[92]However, ChAT deficiency is not believed to be the main cause of this disease.[89]
Amyotrophic lateral sclerosis (ALS or Lou Gehrig's disease)
[edit]Patients withALSshow a marked decrease in ChAT activity in motor neurons in thespinal cordandbrain.[93]Low levels of ChAT activity are an early indication of the disease and are detectable long before motor neurons begin to die. This can even be detected before the patient issymptomatic.[94]
Huntington's disease
[edit]Patients withHuntington'salso show a marked decrease in ChAT production.[95]Though the specific cause of the reduced production is not clear, it is believed that the death of medium-sized motor neurons with spinydendritesleads to the lower levels of ChAT production.[89]
Schizophrenia
[edit]Patients with Schizophrenia also exhibit decreased levels of ChAT, localized to themesopontine tegmentof the brain[96]and thenucleus accumbens,[97]which is believed to correlate with the decreased cognitive functioning experienced by these patients.[89]
Sudden infant death syndrome (SIDS)
[edit]Recent studies have shown thatSIDSinfants show decreased levels of ChAT in both thehypothalamusand thestriatum.[89]SIDS infants also display fewer neurons capable of producing ChAT in the vagus system.[98]These defects in the medulla could lead to an inability to control essentialautonomicfunctions such as thecardiovascularandrespiratorysystems.[98]
Congenital myasthenic syndrome (CMS)
[edit]CMSis a family of diseases that are characterized by defects inneuromuscular transmissionwhich leads to recurrent bouts ofapnea(inability to breathe) that can be fatal.[99]ChAT deficiency is implicated in myasthenia syndromes where the transition problem occurspresynaptically.[100]These syndromes are characterized by the patients’ inability to resynthesizeacetylcholine.[100]
Uses in biotechnology
[edit]Terminal transferases
[edit]Terminal transferasesare transferases that can be used to label DNA or to produceplasmid vectors.[101]It accomplishes both of these tasks by addingdeoxynucleotidesin the form of a template to thedownstreamend or3'end of an existing DNA molecule. Terminal transferase is one of the few DNA polymerases that can function without an RNA primer.[101]
Glutathione transferases
[edit]The family of glutathione transferases (GST) is extremely diverse, and therefore can be used for a number of biotechnological purposes. Plants use glutathione transferases as a means to segregate toxic metals from the rest of the cell.[102]These glutathione transferases can be used to createbiosensorsto detect contaminants such as herbicides and insecticides.[103]Glutathione transferases are also used in transgenic plants to increase resistance to both biotic and abiotic stress.[103]Glutathione transferasesare currently being explored as targets foranti-cancer medicationsdue to their role indrug resistance.[103]Further, glutathione transferase genes have been investigated due to their ability to preventoxidative damageand have shown improved resistance intransgeniccultigens.[104]
Rubber transferases
[edit]Currently the only available commercial source of naturalrubberis theHeveaplant (Hevea brasiliensis). Natural rubber is superior tosynthetic rubberin a number of commercial uses.[105]Efforts are being made to produce transgenic plants capable of synthesizing natural rubber, includingtobaccoandsunflower.[106]These efforts are focused on sequencing the subunits of the rubber transferase enzyme complex in order to transfect these genes into other plants.[106]
Membrane-associated transferases
[edit]Many transferases associate withbiological membranesasperipheral membrane proteinsor anchored to membranes through a singletransmembrane helix,[107]for example numerousglycosyltransferasesinGolgi apparatus.Some others are multi-spantransmembrane proteins,for example certainoligosaccharyltransferasesor microsomalglutathione S-transferasefromMAPEG family.
References
[edit]- ^"EC 2.7.7 Nucleotidyltransferases".Enzyme Nomenclature. Recommendations.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved4 October2020.
- ^"Transferase".Genetics Home Reference.National Institute of Health.Retrieved4 November2013.
- ^Moore SA, Jencks WP (Sep 1982)."Model reactions for CoA transferase involving thiol transfer. Anhydride formation from thiol esters and carboxylic acids".The Journal of Biological Chemistry.257(18): 10882–92.doi:10.1016/S0021-9258(18)33907-3.PMID6955307.
- ^Wishart D."Tryptophan Metabolism".Small Molecule Pathway Database.Department of Computing Science and Biological Sciences, University of Alberta.Retrieved4 November2013.
- ^Herbst EA, MacPherson RE, LeBlanc PJ, Roy BD, Jeoung NH, Harris RA, Peters SJ (Jan 2014)."Pyruvate dehydrogenase kinase-4 contributes to the recirculation of gluconeogenic precursors during postexercise glycogen recovery".American Journal of Physiology. Regulatory, Integrative and Comparative Physiology.306(2): R102–7.doi:10.1152/ajpregu.00150.2013.PMC3921314.PMID24305065.
- ^Watson, James D.Molecular Biology of the Gene.Upper Saddle River, NJ: Pearson, 2013. Print.
- ^Boyce S, Tipton KF (2005). "Enzyme Classification and Nomenclature".Encyclopedia of Life Sciences.doi:10.1038/npg.els.0003893.ISBN978-0470016176.
- ^Morton RK (Jul 1953). "Transferase activity of hydrolytic enzymes".Nature.172(4367): 65–8.Bibcode:1953Natur.172...65M.doi:10.1038/172065a0.PMID13072573.S2CID4180213.
- ^Needham, Dorothy M (1930)."A quantitative study of succinic acid in muscle: Glutamic and aspartic acids as precursors".Biochem J.24(1): 208–27.doi:10.1042/bj0240208.PMC1254374.PMID16744345.
- ^Snell EE, Jenkins WT (December 1959). "The mechanism of the transamination reaction".Journal of Cellular and Comparative Physiology.54(S1): 161–177.doi:10.1002/jcp.1030540413.PMID13832270.
- ^Braunstein AE, Kritzmann MG (1937). "Formation and Breakdown of Amino-acids by Inter-molecular Transfer of the Amino Group".Nature.140(3542): 503–504.Bibcode:1937Natur.140R.503B.doi:10.1038/140503b0.S2CID4009655.
- ^Schoenheimer R (1949).The Dynamic State of Body Constituents.Hafner Publishing Co Ltd.ISBN978-0-02-851800-8.
- ^Guggenheim KY (Nov 1991)."Rudolf Schoenheimer and the concept of the dynamic state of body constituents".The Journal of Nutrition.121(11): 1701–4.doi:10.1093/jn/121.11.1701.PMID1941176.
- ^Hird FJ, Rowsell EV (Sep 1950). "Additional transaminations by insoluble particle preparations of rat liver".Nature.166(4221): 517–8.Bibcode:1950Natur.166..517H.doi:10.1038/166517a0.PMID14780123.S2CID4215187.
- ^Munch-Petersen A, Kalckar HM, Cutolo E, Smith EE (Dec 1953). "Uridyl transferases and the formation of uridine triphosphate; enzymic production of uridine triphosphate: uridine diphosphoglucose pyrophosphorolysis".Nature.172(4388): 1036–7.Bibcode:1953Natur.172.1036M.doi:10.1038/1721036a0.PMID13111246.S2CID452922.
- ^"Physiology or Medicine 1970 - Press Release".Nobelprize.org.Nobel Media AB.Retrieved5 November2013.
- ^Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT (Nov 1996)."A new enzyme superfamily - the phosphopantetheinyl transferases".Chemistry & Biology.3(11): 923–36.doi:10.1016/S1074-5521(96)90181-7.PMID8939709.
- ^Wongtrakul J, Pongjaroenkit S, Leelapat P, Nachaiwieng W, Prapanthadara LA, Ketterman AJ (Mar 2010). "Expression and characterization of three new glutathione transferases, an epsilon (AcGSTE2-2), omega (AcGSTO1-1), and theta (AcGSTT1-1) from Anopheles cracens (Diptera: Culicidae), a major Thai malaria vector".Journal of Medical Entomology.47(2): 162–71.doi:10.1603/me09132.PMID20380296.S2CID23558834.
- ^Sen J, Goltz JS, Stevens L, Stein D (Nov 1998)."Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity".Cell.95(4): 471–81.doi:10.1016/s0092-8674(00)81615-3.PMID9827800.S2CID27722532.
- ^Moussian B, Roth S (Nov 2005)."Dorsoventral axis formation in the Drosophila embryo--shaping and transducing a morphogen gradient".Current Biology.15(21): R887–99.Bibcode:2005CBio...15.R887M.doi:10.1016/j.cub.2005.10.026.PMID16271864.S2CID15984116.
- ^Zhu X, Sen J, Stevens L, Goltz JS, Stein D (Sep 2005)."Drosophila pipe protein activity in the ovary and the embryonic salivary gland does not require heparan sulfate glycosaminoglycans".Development.132(17): 3813–22.doi:10.1242/dev.01962.PMID16049108.
- ^Zhang Z, Stevens LM, Stein D (Jul 2009)."Sulfation of eggshell components by Pipe defines dorsal-ventral polarity in the Drosophila embryo".Current Biology.19(14): 1200–5.Bibcode:2009CBio...19.1200Z.doi:10.1016/j.cub.2009.05.050.PMC2733793.PMID19540119.
- ^Xu D, Song D, Pedersen LC, Liu J (Mar 2007)."Mutational study of heparan sulfate 2-O-sulfotransferase and chondroitin sulfate 2-O-sulfotransferase".The Journal of Biological Chemistry.282(11): 8356–67.doi:10.1074/jbc.M608062200.PMID17227754.
- ^ab"EC 2 Introduction".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved5 November2013.
- ^Shaw WV, Tsai L, Stadtman ER (Feb 1966)."The enzymatic synthesis of N-methylglutamic acid".The Journal of Biological Chemistry.241(4): 935–45.doi:10.1016/S0021-9258(18)96855-9.PMID5905132.
- ^Lower S."Naming Chemical Substances".Chem1 General Chemistry Virtual Textbook.Retrieved13 November2013.
- ^Hausmann R (3 December 2010).To grasp the essence of life: a history of molecular biology.Dordrecht: Springer. pp. 198–199.ISBN978-90-481-6205-5.
- ^"EC 2.7.7.6".IUBMB Enzyme Nomenclature.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved12 November2013.
- ^ab"EC2 Transferase Nomenclature".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved4 November2013.
- ^ab"Transferase".Encyclopædia Britannica.Encyclopædia Britannica, Inc.Retrieved28 July2016.
- ^"EC 2.1.3: Carboxy- and Carbamoyltransferases".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved25 November2013.
- ^"carbamoyltransferase".The Free Dictionary.Farlex, Inc.Retrieved25 November2013.
- ^"carbamoyl group (CHEBI:23004)".ChEBI: The database and ontology of Chemical Entities of Biological Interest.European Molecular Biology Laboratory.Retrieved25 November2013.
- ^Reichard P, Hanshoff G (1956)."Aspartate Carbamyl Transferase fromEscherichia coli"(PDF).Acta Chemica Scandinavica.10:548–566.doi:10.3891/acta.chem.scand.10-0548.
- ^"ENZYME class 2.2.1".ExPASy: Bioinformatics Resource Portal.Swiss Institute of Bioinformatics.Retrieved25 November2013.
- ^"Pentose Phosphate Pathway".Molecular Biochemistry II Notes.The Biochemistry and Biophysics Program at Renssalaer Polytechnic Institute.Retrieved25 November2013.
- ^"EC 2.2.1.2 Transaldolase".Enzyme Structures Database.European Molecular Biology Laboratory.Retrieved25 November2013.
- ^Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V (May 2009)."Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome".Nature Structural & Molecular Biology.16(5): 528–33.doi:10.1038/nsmb.1577.PMC2679717.PMID19363482.
- ^"ENZYME entry: EC 2.3.2.12".ExPASy: Bioinformatics Resource Portal.Swiss Institute of Bioinformatics.Retrieved26 November2013.
- ^"Keyword Glycosyltransferase".UniProt.UniProt Consortium.Retrieved26 November2013.
- ^Fitzgerald DK, Brodbeck U, Kiyosawa I, Mawal R, Colvin B, Ebner KE (Apr 1970)."Alpha-lactalbumin and the lactose synthetase reaction".The Journal of Biological Chemistry.245(8): 2103–8.doi:10.1016/S0021-9258(18)63212-0.PMID5440844.
- ^"ENZYME entry: EC 2.4.1.22".ExPASy: Bioinformatics Resource Portal.Swiss Institute of Bioinformatics.Retrieved26 November2013.
- ^"EC 2.5".IntEnz.European Molecular Biology Laboratory.Retrieved26 November2013.
- ^Qabazard B, Ahmed S, Li L, Arlt VM, Moore PK, Stürzenbaum SR (2013)."C. elegans aging is modulated by hydrogen sulfide and the sulfhydrylase/cysteine synthase cysl-2".PLOS ONE.8(11): e80135.Bibcode:2013PLoSO...880135Q.doi:10.1371/journal.pone.0080135.PMC3832670.PMID24260346.
- ^"EC 2.6.2".IUBMB Enzyme Nomenclatur.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved28 November2013.
- ^Kirsch JF, Eichele G, Ford GC, Vincent MG, Jansonius JN, Gehring H, Christen P (Apr 1984). "Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure".Journal of Molecular Biology.174(3): 497–525.doi:10.1016/0022-2836(84)90333-4.PMID6143829.
- ^"Enzyme entry:2.6.1.1".ExPASy: Bioinformatics Resource Portal.Swiss Institute of Bioinformatics.Retrieved28 November2013.
- ^"EC 2.7".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved4 December2013.
- ^Yee A, Wu L, Liu L, Kobayashi R, Xiong Y, Hall FL (Jan 1996)."Biochemical characterization of the human cyclin-dependent protein kinase activating kinase. Identification of p35 as a novel regulatory subunit".The Journal of Biological Chemistry.271(1): 471–7.doi:10.1074/jbc.271.1.471.PMID8550604.S2CID20348897.
- ^Lewis R (2008).Human genetics: concepts and applications(8th ed.). Boston: McGraw-Hill/Higher Education. p.32.ISBN978-0-07-299539-8.
- ^"ENZYME Entry: EC 2.7.11.22".ExPASy: Bioinformatics Resource Portal.Swiss Institute of Bioinformatics.Retrieved4 December2013.
- ^"1aqy Summary".Protein Data Bank in Europe Bringing Structure to Biology.The European Bioinformatics Institute.Retrieved11 December2013.
- ^"EC 2.8 Transferring Sulfur-Containing Groups".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved11 December2013.
- ^Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, Pedersen LC (Jun 2001)."Structure and function of sulfotransferases".Archives of Biochemistry and Biophysics.390(2): 149–57.doi:10.1006/abbi.2001.2368.PMID11396917.
- ^"EC 2.8 Transferring Sulfur-Containing Groups".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved11 December2013.
- ^"Enzyme 2.8.2.2".Kegg: DBGET.Kyoto University Bioinformatics Center.Retrieved11 December2013.
- ^Ou Z, Shi X, Gilroy RK, Kirisci L, Romkes M, Lynch C, Wang H, Xu M, Jiang M, Ren S, Gramignoli R, Strom SC, Huang M, Xie W (Jan 2013)."Regulation of the human hydroxysteroid sulfotransferase (SULT2A1) by RORα and RORγ and its potential relevance to human liver diseases".Molecular Endocrinology.27(1): 106–15.doi:10.1210/me.2012-1145.PMC3545217.PMID23211525.
- ^Sekura RD, Marcus CJ, Lyon ES, Jakoby WB (May 1979). "Assay of sulfotransferases".Analytical Biochemistry.95(1): 82–6.doi:10.1016/0003-2697(79)90188-x.PMID495970.
- ^"EC 2.9.1".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved11 December2013.
- ^Forchhammer K, Böck A (Apr 1991)."Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence".The Journal of Biological Chemistry.266(10): 6324–8.doi:10.1016/S0021-9258(18)38121-3.PMID2007585.
- ^"EC 2.10.1".School of Biological & Chemical Sciences at Queen Mary, University of London.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB).Retrieved11 December2013.
- ^Nichols JD, Xiang S, Schindelin H, Rajagopalan KV (Jan 2007)."Mutational analysis of Escherichia coli MoeA: two functional activities map to the active site cleft".Biochemistry.46(1): 78–86.doi:10.1021/bi061551q.PMC1868504.PMID17198377.
- ^Wünschiers R, Jahn M, Jahn D, Schomburg I, Peifer S, Heinzle E, Burtscher H, Garbe J, Steen A, Schobert M, Oesterhelt D, Wachtveitl J, Chang A (2010). "Chapter 3: Metabolism". In Michal G, Schomburg D (eds.).Biochemical Pathways: an Atlas of Biochemistry and Molecular Biology(2nd ed.). Oxford: Wiley-Blackwell. p. 140.doi:10.1002/9781118657072.ch3.ISBN9780470146842.
- ^abNishida C, Tomita T, Nishiyama M, Suzuki R, Hara M, Itoh Y, Ogawa H, Okumura K, Nishiyama C (2011)."B-transferase with a Pro234Ser substitution acquires AB-transferase activity".Bioscience, Biotechnology, and Biochemistry.75(8): 1570–5.doi:10.1271/bbb.110276.PMID21821934.
- ^ab"ABO ABO blood group (transferase A, alpha 1-3-N-acetylgalactosaminyltransferase; transferase B, alpha 1-3-galactosyltransferase) [ Homo sapiens (human) ]".NCBI.Retrieved2 December2013.
- ^abcdDatta SP, Smith GH, Campbell PN (2000).Oxford Dictionary of Biochemistry and Molecular Biology(Rev. ed.). Oxford: Oxford Univ. Press.ISBN978-0-19-850673-7.
- ^O'Neil D."ABO Blood Groups".Human Blood: An Introduction to Its Components and Types.Behavioral Sciences Department, Palomar College.Retrieved2 December2013.
- ^"ABO Blood Group (Transferase A, Alpha 1-3-N-Acetylgalactosaminyltransferase;Transferase B, Alpha 1-3-Galactosyltransferase)".GeneCards: The Human Gene Compendium.Weizmann Institute of Science.Retrieved2 December2013.
- ^Moran, Lawrence (2007-02-22)."Human ABO Gene".Retrieved2 December2013.
- ^Kidd, Kenneth."ABO blood group (transferase A, alpha 1-3-N-acetylgalactosaminyltransferase; transferase B, alpha 1-3-galactosyltransferase)".Retrieved2 December2013.
- ^"Succinyl-CoA:3-ketoacid CoA transferase deficiency".Genetics Home Reference.National Institute of Health.Retrieved4 November2013.
- ^abcd"SUCCINYL-CoA:3-OXOACID CoA TRANSFERASE DEFICIENCY".OMIM.Retrieved22 November2013.
- ^"SCOT deficiency".NIH.Retrieved22 November2013.
- ^"Succinyl-CoA 3-Oxoacid Transferase Deficiency"(PDF).Climb National Information Centre.Retrieved22 November2013.
- ^"Carnitine plamitoyltransferase I deficiency".Genetics Home Reference.National Institute of Health.Retrieved4 November2013.
- ^abcdefgWeiser, Thomas (1993)."Carnitine Palmitoyltransferase II Deficiency".NIH.PMID20301431.Retrieved22 November2013.
- ^"Galactosemia".Genetics Home Reference.National Institute of Health.Retrieved4 November2013.
- ^Dobrowolski SF, Banas RA, Suzow JG, Berkley M, Naylor EW (Feb 2003)."Analysis of common mutations in the galactose-1-phosphate uridyl transferase gene: new assays to increase the sensitivity and specificity of newborn screening for galactosemia".The Journal of Molecular Diagnostics.5(1): 42–7.doi:10.1016/S1525-1578(10)60450-3.PMC1907369.PMID12552079.
- ^Murphy M, McHugh B, Tighe O, Mayne P, O'Neill C, Naughten E, Croke DT (Jul 1999)."Genetic basis of transferase-deficient galactosaemia in Ireland and the population history of the Irish Travellers".European Journal of Human Genetics.7(5): 549–54.doi:10.1038/sj.ejhg.5200327.PMID10439960.S2CID22402528.
- ^Mahmood U, Imran M, Naik SI, Cheema HA, Saeed A, Arshad M, Mahmood S (Nov 2012). "Detection of common mutations in the GALT gene through ARMS".Gene.509(2): 291–4.doi:10.1016/j.gene.2012.08.010.PMID22963887.S2CID11479049.
- ^abc"Galactosemia".NORD.Retrieved22 November2013.
- ^Berry GT (2000)."Classic Galactosemia and Clinical Variant Galactosemia".GeneReviews [Internet].PMID20301691.
- ^Bosch AM (Aug 2006). "Classical galactosaemia revisited".Journal of Inherited Metabolic Disease.29(4): 516–25.doi:10.1007/s10545-006-0382-0.PMID16838075.S2CID16382462.
- ^Karadag N, Zenciroglu A, Eminoglu FT, Dilli D, Karagol BS, Kundak A, Dursun A, Hakan N, Okumus N (2013). "Literature review and outcome of classic galactosemia diagnosed in the neonatal period".Clinical Laboratory.59(9–10): 1139–46.doi:10.7754/clin.lab.2013.121235.PMID24273939.
- ^Strauss WL, Kemper RR, Jayakar P, Kong CF, Hersh LB, Hilt DC, Rabin M (Feb 1991)."Human choline acetyltransferase gene maps to region 10q11-q22.2 by in situ hybridization".Genomics.9(2): 396–8.doi:10.1016/0888-7543(91)90273-H.PMID1840566.
- ^Braida D, Ponzoni L, Martucci R, Sparatore F, Gotti C, Sala M (May 2014). "Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish".Psychopharmacology.231(9): 1975–85.doi:10.1007/s00213-013-3340-1.PMID24311357.S2CID8707545.
- ^Stone TW (Sep 1972)."Cholinergic mechanisms in the rat somatosensory cerebral cortex".The Journal of Physiology.225(2): 485–99.doi:10.1113/jphysiol.1972.sp009951.PMC1331117.PMID5074408.
- ^Guzman MS, De Jaeger X, Drangova M, Prado MA, Gros R, Prado VF (Mar 2013)."Mice with selective elimination of striatal acetylcholine release are lean, show altered energy homeostasis and changed sleep/wake cycle".Journal of Neurochemistry.124(5): 658–69.doi:10.1111/jnc.12128.PMID23240572.S2CID22798872.
- ^abcdefOda Y (Nov 1999)."Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system"(PDF).Pathology International.49(11): 921–37.doi:10.1046/j.1440-1827.1999.00977.x.PMID10594838.S2CID23621617.
- ^"Choline O-Acetyltransferase".GeneCards: The Human Gene Compendium.Weizmann Institute of Science.Retrieved5 December2013.
- ^Szigeti C, Bencsik N, Simonka AJ, Legradi A, Kasa P, Gulya K (May 2013)."Long-term effects of selective immunolesions of cholinergic neurons of the nucleus basalis magnocellularis on the ascending cholinergic pathways in the rat: a model for Alzheimer's disease"(PDF).Brain Research Bulletin.94:9–16.doi:10.1016/j.brainresbull.2013.01.007.PMID23357177.S2CID22103097.
- ^González-Castañeda RE, Sánchez-González VJ, Flores-Soto M, Vázquez-Camacho G, Macías-Islas MA, Ortiz GG (Mar 2013)."Neural restrictive silencer factor and choline acetyltransferase expression in cerebral tissue of Alzheimer's Disease patients: A pilot study".Genetics and Molecular Biology.36(1): 28–36.doi:10.1590/S1415-47572013000100005.PMC3615522.PMID23569405.
- ^Rowland LP, Shneider NA (May 2001). "Amyotrophic lateral sclerosis".The New England Journal of Medicine.344(22): 1688–700.doi:10.1056/NEJM200105313442207.PMID11386269.
- ^Casas C, Herrando-Grabulosa M, Manzano R, Mancuso R, Osta R, Navarro X (Mar 2013)."Early presymptomatic cholinergic dysfunction in a murine model of amyotrophic lateral sclerosis".Brain and Behavior.3(2): 145–58.doi:10.1002/brb3.104.PMC3607155.PMID23531559.
- ^Smith R, Chung H, Rundquist S, Maat-Schieman ML, Colgan L, Englund E, Liu YJ, Roos RA, Faull RL, Brundin P, Li JY (Nov 2006)."Cholinergic neuronal defect without cell loss in Huntington's disease".Human Molecular Genetics.15(21): 3119–31.doi:10.1093/hmg/ddl252.PMID16987871.
- ^Karson CN, Casanova MF, Kleinman JE, Griffin WS (Mar 1993). "Choline acetyltransferase in schizophrenia".The American Journal of Psychiatry.150(3): 454–9.doi:10.1176/ajp.150.3.454.PMID8434662.
- ^Mancama D, Mata I, Kerwin RW, Arranz MJ (Oct 2007). "Choline acetyltransferase variants and their influence in schizophrenia and olanzapine response".American Journal of Medical Genetics Part B.144B(7): 849–53.doi:10.1002/ajmg.b.30468.PMID17503482.S2CID6882521.
- ^abMallard C, Tolcos M, Leditschke J, Campbell P, Rees S (Mar 1999)."Reduction in choline acetyltransferase immunoreactivity but not muscarinic-m2 receptor immunoreactivity in the brainstem of SIDS infants".Journal of Neuropathology and Experimental Neurology.58(3): 255–64.doi:10.1097/00005072-199903000-00005.PMID10197817.
- ^Engel AG, Shen XM, Selcen D, Sine S (Dec 2012)."New horizons for congenital myasthenic syndromes".Annals of the New York Academy of Sciences.1275(1): 54–62.Bibcode:2012NYASA1275...54E.doi:10.1111/j.1749-6632.2012.06803.x.PMC3546605.PMID23278578.
- ^abMaselli RA, Chen D, Mo D, Bowe C, Fenton G, Wollmann RL (Feb 2003). "Choline acetyltransferase mutations in myasthenic syndrome due to deficient acetylcholine resynthesis".Muscle & Nerve.27(2): 180–7.doi:10.1002/mus.10300.PMID12548525.S2CID10373463.
- ^abBowen, R."Terminal Transferase".Biotechnology and Genetic Engineering.Colorado State University.Retrieved10 November2013.
- ^Kumar B, Singh-Pareek SL, Sopory SK (2008). "Chapter 23: Glutathione Homeostasis and Abiotic Stresses in Plants: Physiological, Biochemical and Molecular Approaches". In Kumar A, Sopory S (eds.).Recent advances in plant biotechnology and its applications: Prof. Dr. Karl-Hermann Neumann commemorative volume.New Delhi: I.K. International Pub. House.ISBN9788189866099.
- ^abcChronopoulou EG, Labrou NE (2009). "Glutathione transferases: emerging multidisciplinary tools in red and green biotechnology".Recent Patents on Biotechnology.3(3): 211–23.doi:10.2174/187220809789389135.PMID19747150.
- ^Sytykiewicz H (2011)."Expression patterns of glutathione transferase gene (GstI) in maize seedlings under juglone-induced oxidative stress".International Journal of Molecular Sciences.12(11): 7982–95.doi:10.3390/ijms12117982.PMC3233451.PMID22174645.
- ^Shintani D."What is Rubber?".Elastomics.University of Nevada, Reno.Retrieved23 November2013.
- ^ab"Development of Domestic Natural Rubber-Producing Industrial Crops Through Biotechnology".USDA.Retrieved23 November2013.
- ^Superfamilies of single-pass transmembrane transferasesinMembranome database

![{\displaystyle {\ce {X-R + Y}}\quad {\xrightarrow[{\text{ transferase }}]{}}\quad {\ce {X + Y-R}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f7cb2cab1d56dd82329258ba1af69425907bd6f7)

