Metal carbido complex
Ametal carbido complexis acoordination complexthat contains acarbonatom as aligand.They are analogous tometal nitrido complexes.Carbido complexes are a molecular subclass ofcarbides,which are prevalent in organometallic and inorganic chemistry. Carbido complexes represent models for intermediates inFischer–Tropschsynthesis,olefin metathesis,and related catalytic industrial processes.[1]Ruthenium-based carbido complexes are by far the most synthesized and characterized to date. Although, complexes containing chromium, gold, iron, nickel, molybdenum, osmium, rhenium, and tungsten cores are also known. Mixed-metal carbides are also known.[2][3]
Carbido clusters
[edit]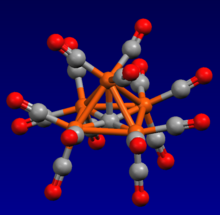
Most molecular carbido complexes are clusters, usually featuring carbide as a six-foldbridging ligand.Examples include [Rh6C(CO)15]2−,and [Ru6C(CO)16]2−.[5]Though exceptions exist, such as the nonanuclear Ruthenium cluster (μ-C)Ru9(CO)14(μ3-η5:η2:η2-C9H7)2,containing a tripped trigonal prism geometry around the carbide.[6]
Theironcarbonyl carbides exist not only in the encapsulated carbon ([Fe6C(CO)16]2−) but also with exposed carbon centres as in Fe5C(CO)15and Fe4C(CO)13.[7]
Bimetallic and exotic clusters such as metal carbide clusterfullerenes (MCCF's) have also been able to be prepared.[8][9]

Doubly bridging carbide ligands
[edit]Bridging carbido ligands can be subdivided into three classes:
- cumulenicLnM=C=M'Ln,
- metallocarbyneLnM≡C−M'Ln,and
- polar covalentLnM≡C:→M'Ln.[10]
Cumulenic compounds generally bridge two metal atoms of the same element and are symmetrical.[11]However, there are exceptions to this.[12]
In contrast, metallocarbyne compounds are generally constitutionally heterobimetallic, with complexes containing varying coordination geometries being common. These moieties have been able to serve as precursors to elaborate molecular scaffolds such as porphyrin derivatives.[13]
The polar covalent class is distinguished from metallocarbynes by a very fine line. This carbide-metal interaction is considered labile in nature. Carbon here can be understood fundamentally as being similar to CO ligands, that is, dative (L-type). Although, this class has also been described to some extent being analogous to the behavior of Lewis acid adduct-forming terminal nitrido and oxo complexes e.g. (PMe2Ph)2Cl-Re≡N-BCl3and tBu(CH2)3(Br)W=O-AlBr3.[14]

Terminal carbides
[edit]In rare cases, carbido ligands are terminal. One example isRuC(PCy3)2Cl2with a Ru-C distance of 163pm,typical for a triple bond.[15]The complex can be obtained by metathesis ofvinyl acetateto give[Ru(CH-p-C6H4Me)(PCy3)2Cl2]results in a metastableRu(Cl2)(PCy3)2C2HOAccomplex, which eliminates acetic acid.[16]
Such transition metal, one coordinate-carbon bonded complexes are comparable to carbon monoxide, cyanide, and isonitrile analogues. These carbides can be used as synthons to access a wide range of carbyne complexes, the most notable being Fischer carbynes.[17]American chemistChristopher C. Cumminsis one of the pioneers of this area.
Preparative routes and characterization
[edit]Carbido clusters
[edit]Synthesis of carbido clusters can be accomplished by hydrolysis, thermolysis of labile ligands, thermal rearrangements, and photolysis. Their synthesis has historically been crudely achieved by serendipitous chance following apparent random molecular organization. One example is the following reaction:
Doubly bridging carbide ligands
[edit]Cumulenic
[edit]Synthetic routes to cumulenic carbido complexes can be efficient and lead to rapid, near quantitative product formation with simple purifications.[18]This dimerization involves the formation of a vinylidene from an alkyne. Mechanistically, there are various proposed pathways, starting with oxidative addition of the alkyne to the metal core, followed by either intramolecular 1,2-H shifts or intermolecular 1,3-H shifts.[19]For Ruthenium coordination complexes, bridging Ru-Cl bond lengths have been observed to lie in the range of 1.76-1.8 Å. Ru-C bonds can vary significantly as a result of trans effect phenomena which is caused by the respective ethylene and vinylidene ligands.
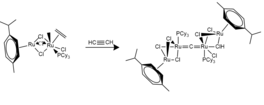

Metallocarbyne
[edit]The appropriate halocarbyne precursors of choice can be reacted withorganolithium reagentsto afford the respective lithiocarbyne derivate by virtue oflithium/halogen exchange.[20]This species can serve as a lynchpin for subsequent carbide linkage with an additional metal complex. Phosphine-based analogues were first introduced by Templeton and co.[21]These types of complexes can be characterized crystallographically and are distinguishable by their Cssymmetry.
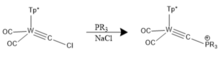
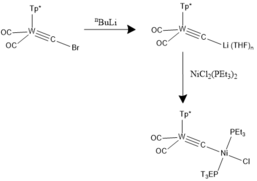
Polar covalent
[edit]Addition of tricyclohexylphosphine to the carbene complex (PPh3)2(Cl)2Ru=C(CHCO2Me)2results in olefin extrusion and yields an air stable anionic carbido complex. This species displaces a dimethyl sulfide ligand from PdCl2(SMe)2to give the μ-carbido bimetallic complex (PCy3)2Cl2Ru≡C-PdCl2(SMe2). Spark towards a novel type of bonding was proposed following empirical observations wherein the carbido-palladium interaction could be readily disturbed. Reversible coordination ensues upon exposure of the bimetallic complex to carbon monoxide. Additionally, no coordination occurs if the anionic carbido complex contains bulky ligands such asH2IMes.This indicates that the thermodynamic sink towards making the C-M bond is not very favorable, suggesting a weak interaction. Although not intuitive, characterization of this type of bonding can be inferred if13C NMR shifts are observed to be far downfield, and C-M bond lengths are similar to those of complexes proven to contain carbon-based σ-donor ligands such as [(Et2H2Im)PdCl(μ-Cl)]2.[22]

Terminal carbido ligands
[edit]Metathesis using Grubbs-type alkylidene complexes can be used to synthesize terminal carbido-containing complexes. One example is RuC(PCy3)2Cl2with a Ru-C distance of 163pm,typical for a triple bond.[23]The complex can be obtained by metathesis ofvinyl acetateto give [Ru(CH-p-C6H4Me)(PCy3)2Cl2] results in a metastable Ru(Cl2)(PCy3)2C2HOAc complex, which eliminates acetic acid.[24]
The "naked" carbido ligand is weakly basic, forming complexes with other metal centers. The C-M bond is typically found to be around 1.65Å.The13C NMRresonance values for the carbido carbons vary widely, but range from δ211-406.[25]Another example of a terminal carbido complex is Li[MoC(NR2)3] (Mo-C distance of 172 pm), which forms upon deprotonation of the respective methylidyne precursor.[26]

See also
[edit]- Metallocarbohedryne( "met-car" ), a stable cluster with formulaM8C12(M = Ti, Zr, V, etc.[which?])
References
[edit]- ^Reinholdt, Anders; Bendix, Jesper (2022-01-12)."Transition Metal Carbide Complexes".Chemical Reviews.122(1): 830–902.doi:10.1021/acs.chemrev.1c00404.ISSN0009-2665.PMID34797626.S2CID244428940.
- ^Zhao, Lili; Chai, Chaoqun; Petz, Wolfgang; Frenking, Gernot (2020-10-26)."Carbones and Carbon Atom as Ligands in Transition Metal Complexes".Molecules.25(21): 4943.doi:10.3390/molecules25214943.ISSN1420-3049.PMC7663554.PMID33114580.
- ^Maitlis, Peter M.; Quyoum, Ruhksana; Long, Helen C.; Turner, Michael L. (October 1999)."Towards a chemical understanding of the Fischer–Tropsch reaction: alkene formation".Applied Catalysis A: General.186(1–2): 363–374.doi:10.1016/S0926-860X(99)00155-6.
- ^Emile H. Braye; Lawrence F. Dahl; Walter. Hubel; Dale L. Wampler (1962). "The Preparation, Properties and Structure of the Iron Carbonyl Carbide Fe5(CO)15C ".J. Am. Chem. Soc.84(24): 4633–4639.doi:10.1021/ja00883a004.
- ^Wang, Ruiyao; Zheng, Zhiping; Koknat, Friedrich W.; Marko, David J.; Müller, Achim; Das, Samar K.; Krickemeyer, Erich; Kuhlmann, Christoph; Therrien, Bruno (2004-04-21), Shapley, John R. (ed.),"Cluster and Polynuclear Compounds",Inorganic Syntheses,Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 184–232,doi:10.1002/0471653683.ch5,ISBN978-0-471-64750-8,retrieved2023-03-25
- ^Chen, Dafa; Mu, Bin; Xu, Shansheng; Wang, Baiquan (September 2006)."Synthesis and structures of the silyl bridged bis(indenyl) diruthenium complexes and a novel indenyl nonanuclear ruthenium cluster Ru9(μ6-C)(CO)14(μ3-η5:η2:η2-C9H7)2".Journal of Organometallic Chemistry.691(18): 3823–3833.doi:10.1016/j.jorganchem.2006.05.030.
- ^Hill, Ernestine W.; Bradley, John S.; Cassidy, Juanita; Whitmire, Kenton H. (2007-01-05), Ginsberg, Alvin P. (ed.),"Tetrairon Carbido Carbonyl Clusters",Inorganic Syntheses,Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 182–188,doi:10.1002/9780470132586.ch36,ISBN978-0-470-13258-6,retrieved2023-03-25
- ^Hill, Ernestine W.; Bradley, John S.; Cassidy, Juanita; Whitmire, Kenton H. (2007-01-05), Ginsberg, Alvin P. (ed.),"Tetrairon Carbido Carbonyl Clusters",Inorganic Syntheses,Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 182–188,doi:10.1002/9780470132586.ch36,ISBN978-0-470-13258-6,retrieved2023-03-25
- ^Saha, Sumit; Zhu, Lei; Captain, Burjor (2013-03-04)."Bimetallic Octahedral Ruthenium–Nickel Carbido Cluster Complexes. Synthesis and Structural Characterization".Inorganic Chemistry.52(5): 2526–2532.doi:10.1021/ic302470w.ISSN0020-1669.PMID23421564.
- ^Hill, Anthony; Sharma, Willis (2012). "Heterodinuclear Bridging Carbido and Phosphocarbyne Complexes".Organometallics.31(7): 2538–2542.doi:10.1021/om201057c.
- ^Mansuy, D. (1980-01-01)."New iron-porphyrin complexes with metal-carbon bond - biological implications".Pure and Applied Chemistry.52(3): 681–690.doi:10.1351/pac198052030681.ISSN1365-3075.S2CID98036930.
- ^Solari, Euro; Antonijevic, Sasa; Gauthier, Sébastien; Scopelliti, Rosario; Severin, Kay (January 2007)."Formation of a Ruthenium μ‐Carbide Complex with Acetylene as the Carbon Source".European Journal of Inorganic Chemistry.2007(3): 367–371.doi:10.1002/ejic.200600991.ISSN1434-1948.
- ^Frogley, Benjamin J.; Hill, Anthony F. (2020)."Carbyne decorated porphyrins".Dalton Transactions.49(35): 12390–12400.doi:10.1039/D0DT02809F.hdl:1885/217011.ISSN1477-9226.PMID32852027.S2CID221347588.
- ^Hejl, Andrew; Trnka, Tina M.; Day, Michael W.; Grubbs, Robert H. (2002)."Terminal ruthenium carbido complexes as σ-donor ligands".Chem. Commun.(21): 2524–2525.doi:10.1039/B207903H.ISSN1359-7345.
- ^Carlson, Robert G.; Gile, Melanie A.; Heppert, Joseph A.; Mason, Mark H.; Powell, Douglas R.; Vander Velde, David; Vilain, Joseph M. (2002). "The Metathesis-Facilitated Synthesis of Terminal Ruthenium Carbide Complexes: A Unique Carbon Atom Transfer Reaction".Journal of the American Chemical Society.124(8): 1580–1581.doi:10.1021/ja017088g.PMID11853424.
- ^Caskey, Stephen (11 November 2005). "Two General Routes to Terminal Carbido Complexes".J. Am. Chem. Soc.127(48): 16750–16751.doi:10.1021/ja0453735.PMID16316197.
- ^Enriquez, Alejandro E.; White, Peter S.; Templeton, Joseph L. (2001-05-01)."Reactions of an Amphoteric Terminal Tungsten Methylidyne Complex".Journal of the American Chemical Society.123(21): 4992–5002.doi:10.1021/ja0035001.ISSN0002-7863.PMID11457327.
- ^Solari, Euro; Antonijevic, Sasa; Gauthier, Sébastien; Scopelliti, Rosario; Severin, Kay (January 2007)."Formation of a Ruthenium μ‐Carbide Complex with Acetylene as the Carbon Source".European Journal of Inorganic Chemistry.2007(3): 367–371.doi:10.1002/ejic.200600991.ISSN1434-1948.
- ^Grotjahn, Douglas B.; Zeng, Xi; Cooksy, Andrew L. (2006-03-01)."Alkyne-to-Vinylidene Transformation on trans -(Cl)Rh(phosphine) 2: Acceleration by a Heterocyclic Ligand and Absence of Bimolecular Mechanism".Journal of the American Chemical Society.128(9): 2798–2799.doi:10.1021/ja058736p.ISSN0002-7863.
- ^Jamison, G. M.; White, P. S.; Templeton, J. L. (June 1991)."Synthesis of Group 6 (aryloxy)carbyne and phosphoniocarbyne complexes from chlorocarbyne precursors".Organometallics.10(6): 1954–1959.doi:10.1021/om00052a048.ISSN0276-7333.
- ^Jamison, G. M.; White, P. S.; Templeton, J. L. (June 1991)."Synthesis of Group 6 (aryloxy)carbyne and phosphoniocarbyne complexes from chlorocarbyne precursors".Organometallics.10(6): 1954–1959.doi:10.1021/om00052a048.ISSN0276-7333.
- ^Liu, Shiuh-Tzung; Hsieh, Tung-Ying; Lee, Gene-Hsiang; Peng, Shie-Ming (1998-03-01)."Carbene Transfer between Transition-Metal Ions".Organometallics.17(6): 993–995.doi:10.1021/om9709897.ISSN0276-7333.
- ^Liu, Shiuh-Tzung; Hsieh, Tung-Ying; Lee, Gene-Hsiang; Peng, Shie-Ming (1998-03-01)."Carbene Transfer between Transition-Metal Ions".Organometallics.17(6): 993–995.doi:10.1021/om9709897.ISSN0276-7333.
- ^Liu, Shiuh-Tzung; Hsieh, Tung-Ying; Lee, Gene-Hsiang; Peng, Shie-Ming (1998-03-01)."Carbene Transfer between Transition-Metal Ions".Organometallics.17(6): 993–995.doi:10.1021/om9709897.ISSN0276-7333.
- ^Hejl, Andrew; Trnka, Tina M.; Day, Michael W.; Grubbs, Robert H. (2002)."Terminal ruthenium carbido complexes as σ-donor ligands".Chem. Commun.(21): 2524–2525.doi:10.1039/B207903H.ISSN1359-7345.
- ^C. Peters, Jonas; L. Odom, Aaron; C. Cummins, Christopher (1997)."A terminal molybdenum carbide prepared by methylidyne deprotonation".Chemical Communications(20): 1995–1996.doi:10.1039/a704251e.ISSN1359-7345.

