Transmembrane protein

Atransmembrane proteinis a type ofintegral membrane proteinthat spans the entirety of thecell membrane.Many transmembrane proteins function asgateways to permit the transportof specific substances across the membrane. They frequently undergo significantconformational changesto move a substance through the membrane. They are usually highlyhydrophobicand aggregate and precipitate in water. They requiredetergentsor nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted usingdenaturing agents.
Thepeptide sequencethat spans the membrane, or thetransmembrane segment,is largely hydrophobic and can be visualized using thehydropathy plot.[1]Depending on the number of transmembrane segments, transmembrane proteins can be classified assingle-pass membrane proteins,or as multipass membrane proteins.[2]Some other integralmembrane proteinsare calledmonotopic,meaning that they are also permanently attached to the membrane, but do not pass through it.[3]
Types
[edit]Classification by structure
[edit]There are two basic types of transmembrane proteins:[4]alpha-helicalandbeta barrels.Alpha-helical proteins are present in the inner membranes of bacterial cells or the plasma membrane of eukaryotic cells, and sometimes in thebacterial outer membrane.[5]This is the major category of transmembrane proteins. In humans, 27% of all proteins have been estimated to be alpha-helical membrane proteins.[6] Beta-barrel proteins are so far found only in outer membranes ofgram-negative bacteria,cell wallsofgram-positive bacteria,outer membranesofmitochondriaandchloroplasts,or can be secreted aspore-forming toxins.All beta-barrel transmembrane proteins have simplest up-and-down topology, which may reflect their common evolutionary origin and similar folding mechanism.[citation needed]
In addition to the protein domains, there are unusual transmembrane elements formed by peptides. A typical example isgramicidin A,a peptide that forms a dimeric transmembrane β-helix.[7]This peptide is secreted bygram-positive bacteriaas anantibiotic.A transmembranepolyproline-II helixhas not been reported in natural proteins. Nonetheless, this structure was experimentally observed in specifically designed artificial peptides.[8]
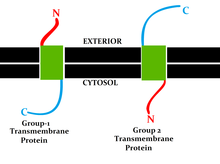
Classification by topology
[edit]This classification refers to theposition of the protein N- and C-termini on the different sidesof thelipid bilayer.Types I, II, III and IV aresingle-pass molecules.Type I transmembrane proteins are anchored to the lipid membrane with a stop-transfer anchor sequence and have their N-terminal domains targeted to theendoplasmic reticulum(ER)lumenduring synthesis (and the extracellular space, if mature forms are located oncell membranes). Type II and III are anchored with a signal-anchor sequence, with type II being targeted to the ER lumen with its C-terminal domain, while type III have their N-terminal domains targeted to the ER lumen. Type IV is subdivided into IV-A, with their N-terminal domains targeted to the cytosol and IV-B, with an N-terminal domain targeted to the lumen.[9]The implications for the division in the four types are especially manifest at the time of translocation and ER-bound translation, when the protein has to be passed through the ER membrane in a direction dependent on the type.[citation needed]
3D structure
[edit]
Membrane proteinstructures can be determined byX-ray crystallography,electron microscopyorNMR spectroscopy.[11]The most commontertiary structuresof these proteins are transmembranehelix bundleandbeta barrel.The portion of the membrane proteins that are attached to the lipid bilayer (seeannular lipid shell) consist mostly of hydrophobic amino acids.[12]
Membrane proteinswhich have hydrophobic surfaces, are relatively flexible and are expressed at relatively low levels. This creates difficulties in obtaining enough protein and then growing crystals. Hence, despite the significant functional importance of membrane proteins, determining atomic resolution structures for these proteins is more difficult than globular proteins.[13]As of January 2013 less than 0.1% of protein structures determined were membrane proteins despite being 20–30% of the total proteome.[14]Due to this difficulty and the importance of this class of proteins methods of protein structure prediction based on hydropathy plots, the positive inside rule and other methods have been developed.[15][16][17]
Thermodynamic stability and folding
[edit]Stability of alpha-helical transmembrane proteins
[edit]Transmembranealpha-helical(α-helical) proteins are unusually stable judging from thermaldenaturationstudies, because they do not unfold completely within the membranes (the complete unfolding would require breaking down too many α-helicalH-bondsin the nonpolar media). On the other hand, these proteins easilymisfold,due to non-native aggregation in membranes, transition to themolten globulestates, formation of non-nativedisulfide bonds,or unfolding of peripheral regions and nonregular loops that are locally less stable.[citation needed]
It is also important to properly define theunfolded state.Theunfolded stateof membrane proteins in detergentmicellesis different from that in the thermaldenaturationexperiments.[citation needed]This state represents a combination of folded hydrophobic α-helices and partially unfolded segments covered by thedetergent.For example, the "unfolded"bacteriorhodopsininSDSmicelles has four transmembrane α-helices folded, while the rest of the protein is situated at the micelle-water interface and can adopt different types of non-nativeamphiphilicstructures. Free energy differences between such detergent-denatured and native states are similar to stabilities of water-soluble proteins (< 10 kcal/mol).[citation needed]
Folding of α-helical transmembrane proteins
[edit]Refolding of α-helical transmembrane proteinsin vitrois technically difficult. There are relatively few examples of the successful refolding experiments, as forbacteriorhodopsin.In vivo,all such proteins are normally folded co-translationally within the large transmembranetranslocon.The translocon channel provides a highly heterogeneous environment for the nascent transmembrane α-helices. A relatively polar amphiphilic α-helix can adopt a transmembrane orientation in the translocon (although it would be at the membrane surface or unfoldedin vitro), because its polar residues can face the central water-filled channel of the translocon. Such mechanism is necessary for incorporation of polar α-helices into structures of transmembrane proteins. The amphiphilic helices remain attached to the translocon until the protein is completely synthesized and folded. If the protein remains unfolded and attached to the translocon for too long, it is degraded by specific "quality control" cellular systems.[citation needed]
Stability and folding of beta-barrel transmembrane proteins
[edit]Stability of beta barrel (β-barrel) transmembrane proteins is similar to stability of water-soluble proteins, based on chemical denaturation studies. Some of them are very stable even in chaotropic agents and high temperature. Their foldingin vivois facilitated by water-solublechaperones,such as protein Skp. It is thought that β-barrel membrane proteins come from one ancestor even having different number of sheets which could be added or doubled during evolution. Some studies show a huge sequence conservation among different organisms and also conserved amino acids which hold the structure and help with folding.[18]
3D structures
[edit]Light absorption-driven transporters
[edit]- Bacteriorhodopsin-like proteins includingrhodopsin(see alsoopsin)
- Bacterialphotosynthetic reaction centresandphotosystemsI and II
- Light-harvesting complexesfrombacteriaandchloroplasts
Oxidoreduction-driven transporters
[edit]- Transmembrane cytochrome b-like proteins:coenzyme Q - cytochrome c reductase(cytochrome bc1 );cytochrome b6f complex;formate dehydrogenase, respiratorynitrate reductase;succinate - coenzyme Q reductase(fumarate reductase); andsuccinate dehydrogenase.Seeelectron transport chain.
- Cytochrome c oxidasesfrombacteriaandmitochondria
Electrochemical potential-driven transporters
[edit]- Proton or sodium translocating F-type and V-typeATPases
P-P-bond hydrolysis-driven transporters
[edit]- P-typecalcium ATPase(five different conformations)
- Calcium ATPase regulatorsphospholambanandsarcolipin
- ABC transporters
- Generalsecretory pathway(Sec)translocon(preprotein translocase SecY)
Porters (uniporters, symporters, antiporters)
[edit]- Mitochondrialcarrier proteins
- Major Facilitator Superfamily (Glycerol-3-phosphate transporter, Lactosepermease,and Multidrug transporter EmrD)
- Resistance-nodulation-cell division(multidrugeffluxtransporter AcrB, seemultidrug resistance)
- Dicarboxylate/amino acid:cation symporter (proton glutamate symporter)
- Monovalent cation/proton antiporter (Sodium/proton antiporter 1 NhaA)
- Neurotransmittersodium symporter
- Ammonia transporters
- Drug/Metabolite Transporter (small multidrug resistance transporter EmrE - the structures are retracted as erroneous)
Alpha-helical channels including ion channels
[edit]- Voltage-gated ion channellike, includingpotassium channelsKcsA and KvAP, andinward-rectifier potassium ion channelKirbac
- Large-conductance mechanosensitive channel, MscL
- Small-conductance mechanosensitive ion channel (MscS)
- CorA metal ion transporters
- Ligand-gated ion channelofneurotransmitterreceptors (acetylcholine receptor)
- Aquaporins
- Chloride channels
- Outer membrane auxiliary proteins (polysaccharide transporter) - α-helical transmembrane proteins from the outer bacterial membrane
Enzymes
[edit]- Methane monooxygenase
- Rhomboid protease
- Disulfide bondformation protein (DsbA-DsbB complex)
Proteins with single transmembrane alpha-helices
[edit]- Subunits ofT cell receptorcomplex
- Cytochrome cnitrite reductasecomplex
- GlycophorinA dimer
- Inovirus (filamentous phage) major coat protein
- Pilin
- Pulmonary surfactant-associated protein
- Monoamine oxidasesA and B
- Fatty acid amide hydrolase[19]
- Cytochrome P450 oxidases
- Corticosteroid 11β-dehydrogenases.
- Signal Peptide Peptidase
Beta-barrels composed of a single polypeptide chain
[edit]- Beta barrels from eight beta-strands and with "shear number" of ten (n=8, S=10). They include:
- Autotransporter domain(n=12,S=14)
- FadL outer membrane protein transport family,includingFatty acidtransporter FadL (n=14,S=14)
- General bacterial porin family,known as trimericporins(n=16,S=20)
- Maltoporin,or sugarporins(n=18,S=22)
- Nucleoside-specific porin(n=12,S=16)
- Outer membrane phospholipase A1(n=12,S=16)
- TonB-dependent receptorsand theirplug domain.They are ligand-gated outer membrane channels (n=22,S=24), includingcobalamintransporter BtuB, Fe(III)-pyochelin receptor FptA, receptor FepA, ferric hydroxamate uptake receptor FhuA, transporter FecA, and pyoverdine receptor FpvA
- Outer membrane protein OpcAfamily (n=10,S=12) that includes outer membraneproteaseOmpT andadhesin/invasin OpcA protein
- Outer membrane protein Gporin family (n=14,S=16)
Note:nandSare, respectively, the number of beta-strands and the "shear number"[20]of thebeta-barrel
Beta-barrels composed of several polypeptide chains
[edit]- Trimeric autotransporter(n=12,S=12)
- Outer membrane efflux proteins,also known as trimeric outer membrane factors (n=12,S=18) including TolC and multidrug resistance proteins
- MspA porin(octamer,n=S=16) and α-hemolysin (heptamern=S=14). These proteins are secreted.
See also
[edit]References
[edit]- ^Manor, Joshua; Feldblum, Esther S.; Arkin, Isaiah T. (2012)."Environment Polarity in Proteins Mapped Noninvasively by FTIR Spectroscopy".The Journal of Physical Chemistry Letters.3(7): 939–944.doi:10.1021/jz300150v.PMC3341589.PMID22563521.
- ^Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002)."Membrane Proteins".Molecular Biology of the Cell. 4th edition.Garland Science.Retrieved31 October2023.
- ^Steven R. Goodman (2008).Medical cell biology.Academic Press. pp. 37–.ISBN978-0-12-370458-0.Retrieved24 November2010.
- ^Jin Xiong (2006).Essential bioinformatics.Cambridge University Press. pp. 208–.ISBN978-0-521-84098-9.Retrieved13 November2010.
- ^alpha-helical proteins in outer membranes includeStanninand certainlipoproteins,and others
- ^Almén MS, Nordström KJ, Fredriksson R, Schiöth HB (2009)."Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin".BMC Biol.7:50.doi:10.1186/1741-7007-7-50.PMC2739160.PMID19678920.
- ^Nicholson, L. K.; Cross, T. A. (1989). "Gramicidin cation channel: an experimental determination of the right-handed helix sense and verification of.beta.-type hydrogen bonding".Biochemistry.28(24): 9379–9385.doi:10.1021/bi00450a019.PMID2482072.
- ^Kubyshkin, Vladimir; Grage, Stephan L.; Ulrich, Anne S.; Budisa, Nediljko (2019)."Bilayer thickness determines the alignment of model polyproline helices in lipid membranes".Physical Chemistry Chemical Physics.21(40): 22396–22408.Bibcode:2019PCCP...2122396K.doi:10.1039/c9cp02996f.PMID31577299.
- ^Harvey Lodish etc.;Molecular Cell Biology,Sixth edition, p.546
- ^Goder, Veit; Spiess, Martin (31 August 2001). "Topogenesis of membrane proteins: determinants and dynamics".FEBS Letters.504(3): 87–93.doi:10.1016/S0014-5793(01)02712-0.PMID11532438.
- ^Cross, Timothy A.; Sharma, Mukesh; Yi, Myunggi; Zhou, Huan-Xiang (2011)."Influence of Solubilizing Environments on Membrane Protein Structures".Trends in Biochemical Sciences.36(2): 117–125.doi:10.1016/j.tibs.2010.07.005.PMC3161620.PMID20724162.
- ^White, Stephen. "General Principle of Membrane Protein Folding and Stability". Stephen White Laboratory Homepage. 10 Nov. 2009. web.[verification needed]
- ^Carpenter, Elisabeth P; Beis, Konstantinos; Cameron, Alexander D; Iwata, So (October 2008)."Overcoming the challenges of membrane protein crystallography".Current Opinion in Structural Biology.18(5): 581–586.doi:10.1016/j.sbi.2008.07.001.PMC2580798.PMID18674618.
- ^"Membrane Proteins of known 3D Structure".Archived fromthe originalon 2013-12-25.Retrieved2016-05-01.
- ^Elofsson, Arne; Heijne, Gunnar von (7 June 2007). "Membrane Protein Structure: Prediction versus Reality".Annual Review of Biochemistry.76(1): 125–140.CiteSeerX10.1.1.332.4023.doi:10.1146/annurev.biochem.76.052705.163539.PMID17579561.
- ^Chen, Chien Peter; Rost, Burkhard (2002). "State-of-the-art in membrane protein prediction".Applied Bioinformatics.1(1): 21–35.CiteSeerX10.1.1.134.7424.PMID15130854.
- ^Hopf, Thomas A.; Colwell, Lucy J.; Sheridan, Robert; Rost, Burkhard; Sander, Chris; Marks, Debora S. (June 2012)."Three-Dimensional Structures of Membrane Proteins from Genomic Sequencing".Cell.149(7): 1607–1621.doi:10.1016/j.cell.2012.04.012.PMC3641781.PMID22579045.
- ^Michalik, Marcin; Orwick-Rydmark, Marcella; Habeck, Michael; Alva, Vikram; Arnold, Thomas; Linke, Dirk; Permyakov, Eugene A. (3 August 2017)."An evolutionarily conserved glycine-tyrosine motif forms a folding core in outer membrane proteins".PLOS ONE.12(8): e0182016.Bibcode:2017PLoSO..1282016M.doi:10.1371/journal.pone.0182016.PMC5542473.PMID28771529.
- ^Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF (November 2002). "Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling".Science.298(5599): 1793–6.Bibcode:2002Sci...298.1793B.doi:10.1126/science.1076535.PMID12459591.S2CID22656813.
- ^Murzin AG, Lesk AM, Chothia C (March 1994). "Principles determining the structure of beta-sheet barrels in proteins. I. A theoretical analysis".J. Mol. Biol.236(5): 1369–81.doi:10.1016/0022-2836(94)90064-7.PMID8126726.
