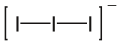Triiodide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Triiodide anion
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| I− 3 | |||
| Molar mass | 380.71341g·mol−1 | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
In chemistry,triiodideusually refers to the triiodide ion,I−
3.This anion, one of thepolyhalogen ions,is composed of threeiodineatoms. It is formed by combiningaqueous solutionsofiodidesalts andiodine.Some salts of the anion have been isolated, includingthallium(I) triiodide(Tl+[I3]−) andammonium triiodide([NH4]+[I3]−). Triiodide is observed to be a red colour in solution.[1]
Nomenclature
[edit]Other chemical compounds with "triiodide" in their name may contain three iodide centers that are not bonded to each other as the triiodide ion, but exist instead as separate iodine atoms or iodide ions. Examples includenitrogen triiodide(NI3) andphosphorus triiodide(PI3), where individual iodine atoms are covalently bonded to a central atom. As some cations have the theoretical possibility to form compounds with both triiodide and iodide ions, such asammonium,compounds containing iodide anions in a 3:1stoichiometricratio should only be referred to as triiodides in cases where the triiodide anion is present. It may also be helpful to indicate the oxidation number of a metal cation, where appropriate. For example, the covalent moleculegallium triiodide(Ga2I6) is better referred to as gallium(III) iodide to emphasise that it is iodide anions that are present, and not triiodide.
Preparation
[edit]The followingexergonicequilibrium gives rise to the triiodideion:
- I2+ I−⇌I−
3
In this reaction, iodide is viewed as aLewis base,and theiodineis aLewis acid.The process is analogous to the reaction ofS8withsodium sulfide(which formspolysulfides) except that the higher polyiodides have branched structures.[2]
Structure and bonding
[edit]The ion is linear and symmetrical. According tovalence shell electron pair repulsion theory,the central iodine atom has three equatorial lone pairs, and the terminal iodine atoms are bonded axially in a linear fashion, due to the three lone pairs bonding to the central iodine-atom. In themolecular orbital model,a common explanation for thehypervalentbonding on the central iodine involves athree-center four-electron bond.The I−I bond is longer than in diatomic iodine,I2.
Inionic compounds,the bond lengths and angles of triiodide vary depending on the nature of thecation.The triiodide anion is easily polarised and in many salts, one I−I bond becomes shorter than the other. Only in combination with large cations, e.g. aquaternary ammoniumsuch as [N(CH3)4]+,may the triiodide remain roughly symmetrical.[3]
Insolutionphase, the bond lengths and angles of triiodide vary depending on the nature ofsolvent.The protic solvents tend to localize the triiodide anion's excess charge, resulting in the triiodide anion's asymmetric structure.[4][5]For example, the triiodide anion in methanol has an asymmetric bent structure with a charge localized on the longer end of the anion.[6]
The dimensions of the triiodide [Ia−Ib−Ic]−bonds in a few sample compounds are shown below:
compound Ia−Ib(pm) Ib−Ic(pm) angle (°) TlI3 306.3 282.6 177.9 RbI3 305.1 283.3 178.11 CsI3 303.8 284.2 178.00 NH4I3 311.4 279.7 178.55 I3−(in methanol)[6] 309.0 296.0 152.0
Properties
[edit]The triiodide ion is the simplestpolyiodide;several higher polyiodides exist. In solution, it appears yellow in low concentrations, and brown at higher concentrations. The triiodide ion is responsible for the well-known blue-black color which arises when iodine solutions interact withstarch.Iodide does not react with starch; nor do solutions of iodine in nonpolarsolvents.
Lugol's iodinecontains potassium iodide and a stoichiometric amount of elemental iodine, so that significant amounts of triiodide ion exist in this solution.Tincture of iodine,although nominally a solution of elemental iodine in ethanol, also contains significant amounts of triiodide, due to its content of both iodide and water.
Photochemistry
[edit]Triiodide is a model system inphotochemistry.Its reaction mechanism has been studied ingasphase,solutionand the solid state. In gas phase, the reaction proceeds in multiplepathwaysthat includeiodinemolecule,metastableions and iodine radicals as photoproducts, which are formed by two-body and three-bodydissociation.[7][8]In condensed phases, due to confinement,geminate recombinationis more common. In solution, only two-body dissociation of triiodide has been observed.[9][10]In the protic solvents, an iodine atom at the shorter end of the triiodide anion dissociates uponphotoexcitationshowing two-body dissociation.[6]In the solid state, the triiodide photochemistry has been studied in compounds involvingquaternary ammonium cations,such astetrabutylammonium triiodide.[11]It has been shown that the solid state photoreaction mechanism depends on the light wavelength, yielding fast recovery in a fewpicoseconds[12]or going through a two-stage process that involves the formation and break-up of a tetraiodideintermediateon longer timescales.[13]Besides, triiodide photochemistry is an important contributor in the environmental cycle ofiodine.[14]Because of the presence of heavyiodineatoms and the well-calibrated chemical pathways, triiodide has also become a computational benchmark system forrelativistic quantum chemistry.[15]
Electrochemistry
[edit]Theredox reactionsof triiodide andiodidehas been proposed as critical steps indye-sensitized solar cells.[16]andrechargeable batteries.[17]
See also
[edit]- Polyiodide
- Tribromide
- Polyhalogen ions
- Three-center four-electron bond
- Iodine–starch test
- Iodometry
- Povidone-iodine
- Lugol's iodine
- Dye-sensitized solar cell
- Organic superconductor
References
[edit]- ^"Halogens as oxidising agents - Chemguide".
- ^Wells, A. F. (1984).Structural Inorganic Chemistry.Oxford: Clarendon Press.ISBN0-19-855370-6.
- ^Atkins; et al. (2010).Inorganic Chemistry(5th ed.). Oxford University Press. p. 431.ISBN978-0-19-923617-6.
- ^Johnson, Alan E.; Myers, Anne B. (1 January 1996)."Solvent Effects in the Raman Spectra of the Triiodide Ion: Observation of Dynamic Symmetry Breaking and Solvent Degrees of Freedom".The Journal of Physical Chemistry.100(19): 7778–7788.doi:10.1021/jp953052x.ISSN0022-3654.
- ^Lynden-Bell, R. M.; Kosloff, R.; Ruhman, S.; Danovich, D.; Vala, J. (8 December 1998)."Does solvation cause symmetry breaking in the I3− ion in aqueous solution?".The Journal of Chemical Physics.109(22): 9928–9937.doi:10.1063/1.477659.ISSN0021-9606.
- ^abcHeo, Jun; Kim, Jong Goo; Choi, Eun Hyuk; Ki, Hosung; Ahn, Doo-Sik; Kim, Jungmin; Lee, Seonggon; Ihee, Hyotcherl (26 January 2022)."Determining the charge distribution and the direction of bond cleavage with femtosecond anisotropic x-ray liquidography".Nature Communications.13(1): 522.Bibcode:2022NatCo..13..522H.doi:10.1038/s41467-022-28168-0.ISSN2041-1723.PMC8792042.PMID35082327.
- ^Hoops, Alexandra A.; Gascooke, Jason R.; Faulhaber, Ann Elise; Kautzman, Kathryn E.; Neumark, Daniel M. (2004-04-03)."Two- and three-body photodissociation of gas phase I3−".The Journal of Chemical Physics.120(17): 7901–7909.Bibcode:2004JChPh.120.7901H.doi:10.1063/1.1691017.hdl:2440/34955.ISSN0021-9606.PMID15267705.S2CID94632820.
- ^Nakanishi, Ryuzo; Saitou, Naoya; Ohno, Tomoyo; Kowashi, Satomi; Yabushita, Satoshi; Nagata, Takashi (2007-05-28)."Photodissociation of gas-phase I3−: Comprehensive understanding of nonadiabatic dissociation dynamics".The Journal of Chemical Physics.126(20): 204311.Bibcode:2007JChPh.126t4311N.doi:10.1063/1.2736691.ISSN0021-9606.PMID17552766.
- ^Banin, Uri; Ruhman, Sanford (1993-03-15)."Ultrafast photodissociation of I 3. Coherent photochemistry in solution".The Journal of Chemical Physics.98(6): 4391–4403.Bibcode:1993JChPh..98.4391B.doi:10.1063/1.465066.ISSN0021-9606.
- ^Kühne, Thomas; Vöhringer, Peter (1996-12-22)."Vibrational relaxation and geminate recombination in the femtosecond-photodissociation of triiodide in solution".The Journal of Chemical Physics.105(24): 10788–10802.Bibcode:1996JChPh.10510788K.doi:10.1063/1.472887.ISSN0021-9606.
- ^Herbstein, F. H.; Kaftory, M.; Kapon, M.; Saenger, W. (1981-01-01). Herbstein, F. H.; Kaftory, M.; Kapon, M.; Saenger, W. (eds.)."Structures of three crystals containing approximately — linear chains of triiodide ions".Zeitschrift für Kristallographie - Crystalline Materials.154(1–2): 11–30.Bibcode:1981ZK....154...11H.doi:10.1524/zkri.1981.154.1-2.11.ISSN2194-4946.
- ^Poulin, Peter R.; Nelson, Keith A. (2006-09-22)."Irreversible Organic Crystalline Chemistry Monitored in Real Time".Science.313(5794): 1756–1760.Bibcode:2006Sci...313.1756P.doi:10.1126/science.1127826.ISSN0036-8075.PMID16946037.S2CID35002522.
- ^Xian, Rui; Corthey, Gastón; Rogers, David M.; Morrison, Carole A.; Prokhorenko, Valentyn I.; Hayes, Stuart A.; Miller, R. J. Dwayne (2017-03-27)."Coherent ultrafast lattice-directed reaction dynamics of triiodide anion photodissociation".Nature Chemistry.9(6): 516–522.Bibcode:2017NatCh...9..516X.doi:10.1038/nchem.2751.hdl:20.500.11820/52dbea74-99b4-454b-aac2-56c7be20947b.ISSN1755-4330.PMID28537597.
- ^Raso, Angela R. W.; Custard, Kyle D.; May, Nathaniel W.; Tanner, David; Newburn, Matt K.; Walker, Lawrence; Moore, Ronald J.; Huey, L. G.; Alexander, Liz; Shepson, Paul B.; Pratt, Kerri A. (2017-09-19)."Active molecular iodine photochemistry in the Arctic".Proceedings of the National Academy of Sciences.114(38): 10053–10058.Bibcode:2017PNAS..11410053R.doi:10.1073/pnas.1702803114.ISSN0027-8424.PMC5617258.PMID28874585.
- ^Gomes, André Severo Pereira; Visscher, Lucas; Bolvin, Hélène; Saue, Trond; Knecht, Stefan; Fleig, Timo; Eliav, Ephraim (2010-08-14)."The electronic structure of the triiodide ion from relativistic correlated calculations: A comparison of different methodologies".The Journal of Chemical Physics.133(6): 064305.Bibcode:2010JChPh.133f4305G.doi:10.1063/1.3474571.hdl:20.500.12210/35342.ISSN0021-9606.PMID20707568.S2CID8849684.
- ^Gibson, Elizabeth A.; Le Pleux, Loïc; Fortage, Jérôme; Pellegrin, Yann; Blart, Errol; Odobel, Fabrice; Hagfeldt, Anders; Boschloo, Gerrit (2012-04-17)."Role of the Triiodide/Iodide Redox Couple in Dye Regeneration in p-Type Dye-Sensitized Solar Cells".Langmuir.28(15): 6485–6493.doi:10.1021/la300215q.ISSN0743-7463.PMID22432412.
- ^Ma, Jizhen; Liu, Miaomiao; He, Yulong; Zhang, Jintao (2021-02-17)."Iodine Redox Chemistry in Rechargeable Batteries".Angewandte Chemie International Edition.60(23): 12636–12647.doi:10.1002/anie.202009871.ISSN1433-7851.PMID32939916.S2CID221769817.