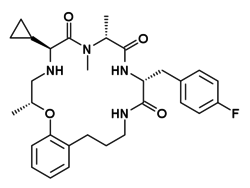
Ulimorelin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C30H39FN4O4 |
| Molar mass | 538.664g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ulimorelin(INN,USAN) (developmental code nameTZP-101) is adrugwith a modifiedcyclicpeptidestructurewhich acts as a selectiveagonistof theghrelin/growth hormone secretagogue receptor(GHSR-1a).[1]Unlike many related drugs, ulimorelin has little or no effect ongrowth hormone(GH)releasein rats.[2]However, like ghrelin and other ghrelin agonists, ulimorelin does stimulate GH release with concomitant increases in insulin-like growth factor 1 (IGF-1) in humans.[3]It has been researched for enhancinggastrointestinal motility,especially ingastroparesis[4]and in aiding recovery of bowel function followinggastrointestinal surgery,whereopioidanalgesicdrugs used for post-operative pain relief may worsen existingconstipation.[5][6][7][8]While ulimorelin has been shown to increase both upper and lower gastrointestinal motility in rats,[8]and showed promising results initially in humans,[4][6]it failed in pivotal clinical trials in post operativeileus.[7]
A commonside effectof ghrelin isreduced blood pressure.Ulimorelin has been shown to inhibitvasoconstrictionof rat arteries in vitro elicited by theα1-adrenoceptorsagonistsphenylephrineandmethoxamine,and to increase artery tension at high concentrations.[9]Effects on blood pressure, however, were not observed in human clinical trials.[4][7]
References
[edit]- ^Hoveyda HR, Marsault E, Gagnon R, Mathieu AP, Vézina M, Landry A, et al. (December 2011). "Optimization of the potency and pharmacokinetic properties of a macrocyclic ghrelin receptor agonist (Part I): Development of ulimorelin (TZP-101) from hit to clinic".Journal of Medicinal Chemistry.54(24): 8305–20.doi:10.1021/jm2007062.PMID22106937.
- ^Fraser GL, Hoveyda HR, Tannenbaum GS (December 2008)."Pharmacological demarcation of the growth hormone, gut motility and feeding effects of ghrelin using a novel ghrelin receptor agonist".Endocrinology.149(12): 6280–8.doi:10.1210/en.2008-0804.PMID18719021.
- ^Lasseter KC, Shaughnessy L, Cummings D, Pezzullo JC, Wargin W, Gagnon R, et al. (February 2008). "Ghrelin agonist (TZP-101): safety, pharmacokinetics and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study".Journal of Clinical Pharmacology.48(2): 193–202.doi:10.1177/0091270007310380.PMID18199894.S2CID206433703.
- ^abcWargin W, Thomas H, Clohs L, St-Louis C, Ejskjaer N, Gutierrez M, et al. (2009). "Contribution of protein binding to the pharmacokinetics of the ghrelin receptor agonist TZP-101 in healthy volunteers and adults with symptomatic gastroparesis: two randomized, double-blind studies and a binding profile study".Clinical Drug Investigation.29(6): 409–18.doi:10.2165/00044011-200929060-00004.PMID19432500.S2CID23063963.
- ^Popescu I, Fleshner PR, Pezzullo JC, Charlton PA, Kosutic G, Senagore AJ (February 2010). "The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial".Diseases of the Colon and Rectum.53(2): 126–34.doi:10.1007/DCR.0b013e3181b54166.PMID20087086.S2CID25357270.
- ^abGreenwood-Van Meerveld B, Kriegsman M, Nelson R (November 2011). "Ghrelin as a target for gastrointestinal motility disorders".Peptides.32(11): 2352–6.doi:10.1016/j.peptides.2011.03.014.PMID21453735.S2CID22222190.
- ^abcBochicchio G, Charlton P, Pezzullo JC, Kosutic G, Senagore A (January 2012)."Ghrelin agonist TZP-101/ulimorelin accelerates gastrointestinal recovery independently of opioid use and surgery type: covariate analysis of phase 2 data".World Journal of Surgery.36(1): 39–45.doi:10.1007/s00268-011-1335-9.PMC3243849.PMID22072430.
- ^abShaw M, Pediconi C, McVey D, Mondou E, Quinn J, Chamblin B, Rousseau F (July 2013). "Safety and efficacy of ulimorelin administered postoperatively to accelerate recovery of gastrointestinal motility following partial bowel resection: results of two randomized, placebo-controlled phase 3 trials".Diseases of the Colon and Rectum.56(7): 888–97.doi:10.1097/DCR.0b013e31829196d0.PMID23739196.S2CID20364202.
- ^Fraser GL, Venkova K, Hoveyda HR, Thomas H, Greenwood-Van Meerveld B (February 2009). "Effect of the ghrelin receptor agonist TZP-101 on colonic transit in a rat model of postoperative ileus".European Journal of Pharmacology.604(1–3): 132–7.doi:10.1016/j.ejphar.2008.12.011.PMID19121631.
