Uranium hexafluoride
You can helpexpand this article with text translated fromthe corresponding articlein German.(November 2012)Click [show] for important translation instructions.
|
| |
| |
| |
| Names | |
|---|---|
| IUPAC names
Uranium hexafluoride
Uranium(VI) fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | hex |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.116 |
| EC Number |
|
| 2923 | |
PubChemCID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2978 (<1%235U) 2977 (>1%235U) |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| UF6 | |
| Molar mass | 352.02 g/mol |
| Appearance | Colorless solid |
| Density | 5.09g/cm3,solid |
| Boiling point | 56.5 °C (133.7 °F; 329.6 K) (sublimes, at atmospheric pressure) |
| Hydrolyzes | |
| Solubility |
|
| Structure | |
| Orthorhombic,oP28 | |
| Pnma, No. 62 | |
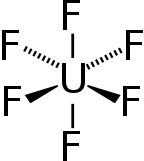
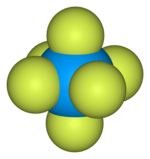
| Octahedral (Oh) | |
| 0 | |
| Thermochemistry | |
Std molar
entropy(S⦵298) |
|
Std enthalpy of
formation(ΔfH⦵298) |
|
| Hazards | |
| Occupational safety and health(OHS/OSH): | |
Main hazards
|
Toxic, corrosive, radioactive[3] |
| GHSlabelling: | |
  
| |
| Danger | |
| H300,H330,H373,H411 | |
| NFPA 704(fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet(SDS) | ICSC 1250 |
| Related compounds | |
Otheranions
|
Uranium hexachloride |
Othercations
|
|
Related uranium fluorides
|
|
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Uranium hexafluoride,sometimes calledhex,is aninorganic compoundwith the formulaUF6.Uranium hexafluoride is a volatile and toxic white solid thatreacts with water,releasing corrosivehydrofluoric acid.The compound reacts mildly withaluminium,forming a thin surface layer ofAlF3that resists any further reaction from the compound.UF6is used in the process ofenriching uranium,which produces fuel fornuclear reactorsandnuclear weapons.
Preparation[edit]
Milled uranium ore—U3O8or "yellowcake"—is dissolved innitric acid,yielding a solution ofuranyl nitrateUO2(NO3)2.Pure uranyl nitrate is obtained bysolvent extraction,then treated withammoniato produceammonium diuranate( "ADU",[NH4]2U2O7). Reduction withhydrogengivesUO2,which is converted withhydrofluoric acid(HF) touranium tetrafluoride,UF4.Oxidation withfluorineyieldsUF6.
TheHoneywell Uranium Hexafluoride Processing Facilityuses a different process.
Duringnuclear reprocessing,uranium is reacted withchlorine trifluorideto giveUF6:
- U + 2 ClF3→ UF6+ Cl2
Properties[edit]
Physical properties[edit]
Atatmospheric pressure,UF6sublimesat 56.5 °C.[4]

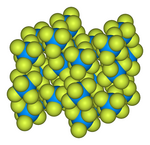
The solid-state structure was determined byneutron diffractionat 77 K and 293 K.[5][6]
-
Ball-and-stick modelof the unit cell of uranium hexafluoride[7]
-
Bond lengths and angles of gaseous uranium hexafluoride[8]
Chemical properties[edit]
It has been shown that uranium hexafluoride is anoxidant[9]and aLewis acidthat is able to bind tofluoride;for instance, the reaction ofcopper(II) fluoridewith uranium hexafluoride inacetonitrileis reported to form copper(II) heptafluorouranate(VI),Cu2+[UF−7]2.[10]
Polymericuranium(VI) fluorides containing organic cations have been isolated and characterized by X-ray diffraction.[11]
Application in the fuel cycle[edit]
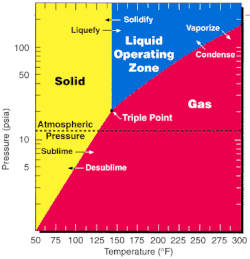
As one of the mostvolatilecompounds of uranium, uranium hexafluoride is relatively convenient to process and is used in both of the main uraniumenrichmentmethods, namelygaseous diffusionand thegas centrifugemethod. Since thetriple pointofUF6;64 °C(147 °F; 337 K) and 152 kPa (22 psi; 1.5 atm);[12]is close to ambient conditions, phase transitions can be achieved with littlethermodynamic work.
Fluorine has only a single naturally occurring stable isotope, soisotopologuesofUF6differ in their molecular weight based solely on the uraniumisotopepresent.[13]This difference is the basis for the physical separation of isotopes in enrichment.
All the other uranium fluorides are nonvolatile solids that arecoordination polymers.
The conversion factor for the238Uisotopologue ofUF6( "hex" ) to "U mass" is 0.676.[14]
Gaseous diffusion requires about 60 times as much energy as the gas centrifuge process: gaseous diffusion-produced nuclear fuel produces 25 times more energy than is used in the diffusion process, while centrifuge-produced fuel produces 1,500 times more energy than is used in the centrifuge process.
In addition to its use in enrichment, uranium hexafluoride has been used in an advanced reprocessing method (fluoride volatility), which was developed in theCzech Republic.In this process,spent nuclear fuelis treated with fluorine gas to transform the oxides or elemental metals into a mixture of fluorides. This mixture is then distilled to separate the different classes of material. Somefission productsform nonvolatile fluorides which remain as solids and can then either be prepared for storage as nuclear waste or further processed either bysolvation-based methods orelectrochemically.
Uranium enrichment produces large quantities ofdepleted uranium hexafluoride(DUF6or D-UF6) as a waste product. The long-term storage of D-UF6presents environmental, health, and safety risks because of its chemical instability. WhenUF6is exposed to moist air, it reacts with the water in the air to produceUO2F2(uranyl fluoride) and HF (hydrogen fluoride) both of which are highly corrosive and toxic. In 2005, 686,500 tonnes of D-UF6was housed in 57,122 storage cylinders located nearPortsmouth, Ohio;Oak Ridge, Tennessee;andPaducah, Kentucky.[15][16]Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated lifetime of the steel cylinders is measured in decades.[17]
Accidents and disposal[edit]
There have been several accidents involving uranium hexafluoride in the US, including a cylinder-filling accident and material release at theSequoyah Fuels Corporationin 1986 where an estimated 29 500 pounds of gaseousUF6escaped.[18][19]The U.S. government has been converting DUF6to soliduranium oxidesfor disposal.[20]Such disposal of the entire DUF6stockpile could cost anywhere from $15 million to $450 million.[21]
References[edit]
- ^"Uranium Hexafluoride".Archived fromthe originalon 2013-09-16.Retrieved2013-08-08.
- ^abcdJohnson, Gerald K. (1979). "The Enthalpy of Formation of Uranium Hexafluoride".The Journal of Chemical Thermodynamics.11(5): 483–490.doi:10.1016/0021-9614(79)90126-5.
- ^Uranium(VI) fluoride
- ^Brickwedde, Ferdinand G.; Hoge, Harold J.; Scott, Russell B. (1948)."The Low Temperature Heat Capacities, Enthalpies, and Entropies of UF4and UF6".J. Chem. Phys.16(5): 429–436.Bibcode:1948JChPh..16..429B.doi:10.1063/1.1746914.
- ^J. H. Levy; John C. Taylor; Paul W. Wilson (1976). "Structure of Fluorides. Part XII. Single-Crystal Neutron Diffraction Study of Uranium Hexafluoride at 293 K".J. Chem. Soc., Dalton Trans.(3): 219–224.doi:10.1039/DT9760000219.
- ^J. H. Levy, J. C. Taylor and A. B. Waugh (1983). "Neutron Powder Structural Studies of UF6,MoF6and WF6at 77 K ".Journal of Fluorine Chemistry.23:29–36.doi:10.1016/S0022-1139(00)81276-2.
- ^J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6:a neutron diffraction analysis ",Acta Crystallogr.,1973,B29,p. 7–12;doi:10.1107/S0567740873001895.
- ^Kimura, Masao; Schomaker, Werner; Smith, Darwin W.; Bernard (1968)."Electron-Diffraction Investigation of the Hexafluorides of Tungsten, Osmium, Iridium, Uranium, Neptunium, and Plutonium".J. Chem. Phys.48(8): 4001–4012.Bibcode:1968JChPh..48.4001K.doi:10.1063/1.1669727.Archived fromthe originalon 2023-01-11.Retrieved2020-10-10.
- ^G. H. Olah; J. Welch (1978). "Synthetic methods and reactions. 46. Oxidation of organic compounds with uranium hexafluoride in haloalkane solutions".J. Am. Chem. Soc.100(17): 5396–5402.doi:10.1021/ja00485a024.
- ^J. A. Berry; R. T. Poole; A. Prescott; D. W. A. Sharp; J. M. Winfield (1976). "The oxidising and fluoride ion acceptor properties of uranium hexafluoride in acetonitrile".J. Chem. Soc., Dalton Trans.(3): 272–274.doi:10.1039/DT9760000272.
- ^S. M. Walker; P. S. Halasyamani; S. Allen; D. O'Hare (1999). "From Molecules to Frameworks: Variable Dimensionality in the UO2(CH3COO)2·2H2O/HF(aq)/Piperazine System. Syntheses, Structures, and Characterization of Zero-Dimensional (C4N2H12)UO2F4·3H2O, One-Dimensional (C4N2H12)2U2F12·H2O, Two-Dimensional (C4N2H12)2(U2O4F5)4·11H2O, and Three-Dimensional (C4N2H12)U2O4F6".J. Am. Chem. Soc.121(45): 10513–10521.doi:10.1021/ja992145f.
- ^"Uranium Hexafluoride: Source: Appendix A of the PEIS (DOE/EIS-0269): Physical Properties".web.evs.anl.gov.Retrieved2022-08-18.
- ^"Uranium Enrichment and the Gaseous Diffusion Process".USEC Inc. Archived fromthe originalon 2007-10-19.Retrieved2007-09-24.
- ^"Unit converter molar mass calculator".TranslatorsCafé.Mississauga, Ontario, Canada: ANVICA Software Development. 1 February 2021.
- ^"How much depleted uranium hexafluoride is stored in the United States?".Depleted UF6FAQs.Argonne National Laboratory.
- ^"Depleted UF6Management Program Documents ".Archivedfrom the original on 2008-02-16.Retrieved2006-05-17.
- ^"What is DUF6?Is it dangerous and what should we do with it? ".Institute for Energy and Environmental Research. 2007-09-24.
- ^Brugge, D.; Delemos, J. L.; Bui, C. (2007)."The Sequoyah Corporation Fuels Release and the Church Rock Spill: Unpublicized Nuclear Releases in American Indian Communities".American Journal of Public Health.97(9): 1595–1600.doi:10.2105/AJPH.2006.103044.PMC1963288.PMID17666688.
- ^ "Have there been accidents involving uranium hexafluoride?".Depleted UF6FAQs.Argonne National Laboratory. Archived fromthe originalon 2017-06-09.
- ^"What is going to happen to the uranium hexafluoride stored in the United States?".Depleted UF6FAQs.Argonne National Laboratory.
- ^"Are there any currently-operating disposal facilities that can accept all of the depleted uranium oxide that would be generated from conversion of DOE's depleted UF6inventory? ".Depleted UF6FAQs.Argonne National Laboratory.
Further reading[edit]
- Gmelins Handbuch der anorganischen Chemie,System Nr. 55, Uran, Teil A, p. 121–123.
- Gmelins Handbuch der anorganischen Chemie,System Nr. 55, Uran, Teil C 8, p. 71–163.
- R. DeWitt:Uranium hexafluoride: A survey of the physico-chemical properties,Technical report, GAT-280; Goodyear Atomic Corp., Portsmouth, Ohio; 12. August 1960;doi:10.2172/4025868.
- Ingmar Grenthe, Janusz Drożdżynński, Takeo Fujino, Edgar C. Buck,Thomas E. Albrecht-Schmitt,Stephen F. Wolf:UraniumArchived2012-01-18 at theWayback Machine,in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.):The Chemistry of the Actinide and Transactinide Elements,Springer, Dordrecht 2006;ISBN1-4020-3555-1,p. 253–698;doi:10.1007/1-4020-3598-5_5(p. 530–531, 557–564).
- US-Patent 2535572:Preparation of UF6;26. December 1950.
- US-Patent 5723837:Uranium Hexafluoride Purification;3. March 1998.
External links[edit]
- Simon Cotton (Uppingham School, Rutland, UK):Uranium Hexafluoride.
- Uranium Hexafluoride (UF6) – Physical and chemical properties of UF6,and its use in uranium processing – Uranium Hexafluoride and Its Properties
- Uranium Hexafluorideat WebElements
- Import of Western depleted uranium hexafluoride (uranium tails) to Russia[dead link 30 June 2017]


![Ball-and-stick model of the unit cell of uranium hexafluoride[7]](https://upload.wikimedia.org/wikipedia/commons/thumb/c/c6/Uranium-hexafluoride-unit-cell-3D-balls.png/180px-Uranium-hexafluoride-unit-cell-3D-balls.png)
![Bond lengths and angles of gaseous uranium hexafluoride[8]](https://upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Uranium_hexafluoride_dimensions.svg/180px-Uranium_hexafluoride_dimensions.svg.png)


