Vanadinite
| Vanadinite | |
|---|---|
 | |
| General | |
| Category | Vanadate minerals Apatitegroup |
| Formula (repeating unit) | Pb5(VO4)3Cl |
| IMA symbol | Vna[1] |
| Strunz classification | 8.BN.05 |
| Crystal system | Hexagonal |
| Crystal class | Dipyramidal (6/m) H-M symbol:(6/m) |
| Space group | P63/m |
| Unit cell | a = 10.3174, c = 7.3378 [Å]; Z = 2 |
| Identification | |
| Formula mass | 1416.27 g/mol |
| Colour | Bright red, orange-red, red-brown, brown, yellow, whitish, grey or colourless or weakly tinted in transmitted light; pale straw-yellow;. may be concentrically zoned |
| Crystal habit | Prismatic or nodular; may be acicular, hairlike, fibrous; rarely rounded, globular |
| Cleavage | None |
| Fracture | Uneven to conchoidal |
| Tenacity | Brittle |
| Mohs scalehardness | 3–4 |
| Lustre | Resinous to sub-adamantine |
| Streak | brownish yellow |
| Diaphaneity | Transparent, translucent or opaque |
| Specific gravity | 6.8–7.1 (measured) 6.95 (calculated) |
| Optical properties | Uniaxial (−) |
| Refractive index | nω= 2.416, nε= 2.350 |
| Birefringence | δ = 0.066 |
| Ultravioletfluorescence | None |
| Melting point | 3,470 °F (1,910 °C) |
| References | [2][3][4] |
Vanadiniteis amineralbelonging to theapatitegroup ofphosphates,with the chemical formulaPb5(VO4)3Cl.It is one of the main industrial ores of the metalvanadiumand a minor source oflead.A dense, brittle mineral, it is usually found in the form of red hexagonalcrystals.It is an uncommon mineral, formed by the oxidation of lead ore deposits such asgalena.First discovered in 1801 inMexico,vanadinite deposits have since been unearthed in South America, Europe, Africa, and North America.
Origins
[edit]Vanadinite is an uncommon mineral, only occurring as the result of chemical alterations to a pre-existing material. It is therefore known as a secondary mineral. It is found inaridclimates and forms byoxidationof primary lead minerals. Vanadinite is especially found in association with the lead sulfide,galena.Other associated minerals includewulfenite,limonite,andbarite.[3][5]
It was originally discovered inMexicoby the Spanish mineralogistAndrés Manuel del Ríoin 1801. He called the mineral "brown lead" and asserted that it contained a new element, which he first named pancromium and later, erythronium. However, he was later led to believe that this was not a new element but merely an impure form of chromium. In 1830,Nils Gabriel Sefströmdiscovered a new element, which he named vanadium. It was later revealed that this was identical to the metal discovered earlier by Andrés Manuel del Río. Del Río's "brown lead" was also rediscovered, in 1838 in Zimapan,Hidalgo,Mexico, and was named vanadinite because of its high vanadium content. Other names that have since been given to vanadinite are johnstonite and lead vanadate.[6]
Occurrence
[edit]Vanadinite occurs as a secondary mineral in theoxidized zoneof lead-bearing deposits; the vanadium is leached from wall-rocksilicates.Associated minerals includemimetite,pyromorphite,descloizite,mottramite,wulfenite,cerussite,anglesite,calcite,barite,and variousiron oxideminerals.[4]
Deposits of vanadinite are found worldwide includingAustria,Spain,Scotland,theUral Mountains,South Africa,Namibia,Morocco,Argentina,Mexico,and four states of theUnited States:Arizona,Colorado,New Mexico,andSouth Dakota.[3][5][7]
Vanadinite deposits are found in over 400 mines across the world. Notable vanadinite mines include those atMibladenand Touisset in Morocco;Tsumeb,Namibia;Cordoba,Argentina; andSierra County,New Mexico, andGila County, Arizona,in the United States.[8]
Structure
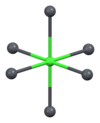
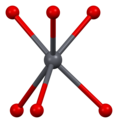
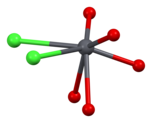
[edit]Vanadinite is a lead chlorovanadate with the chemical formula Pb5(VO4)3Cl. It is composed (by weight) of 73.15% lead, 10.79% vanadium, 13.56% oxygen, and 2.50% chlorine. Each structural unit of vanadinite contains a chlorine ion surrounded by sixdivalentlead ions at the corners of a regularoctahedron,with one of the lead ions provided by an adjoining vanadinite molecule. The distance between each lead and chlorine ion is 317picometres.The shortest distance between each lead ion is 4.48 Å. The octahedron shares two of its opposite faces with that of neighbouring vanadinite units, forming a continuous chain of octahedrons. Each vanadium atom is surrounded by four oxygen atoms at the corners of an irregulartetrahedron.The distance between each oxygen and vanadium atom is either 1.72 or 1.76 Å. Three oxygen tetrahedrons adjoin each of the lead octahedrons along the chain.[2][9][10]
Crystals of vanadinite conform to ahexagonalsystem ofsymmetry.This internal structure is often reflected in the hexagonal external shape of the crystals. The crystals are usually in the form of short hexagonal prisms, but can also be found as hexagonal pyramids, rounded masses or crusts. Aunit cellof vanadinite, the smallest divisible unit that possesses the same symmetry and properties, is in the form of a hexagonal prism. The unit cell of vanadinite is composed of two of its molecules and has the dimensionsa= 10.331Åandc= 7.343 Å, whereais the length of each side of the hexagon andcis the height of the prism. The volume of each unit cell of vanadinite, given by the formula V =a2csin(60°), is 678.72 Å3.[2][5]
Characteristics
[edit]Vanadinite is in theapatitegroup ofphosphates,and forms a chemical series with the mineralspyromorphite(Pb5(PO4)3Cl) andmimetite(Pb5(AsO4)3Cl), with both of which it may formsolid solutions.Whereas most chemical series involve the substitution of metallic ions, this series substitutes its anion groups; phosphate (PO4),arsenate(AsO4) andvanadate(VO4). Commonimpuritiesof vanadinite includephosphorus,arsenicandcalcium,where these may act as anisomorphicsubstitute for vanadium (in the first two cases) or lead (in the second). Vanadinite when containing a high amount of the arsenic impurity is known asendlichite.[3][5]
Vanadinite is usually bright-red or orange-red in colour, although sometimes brown, red-brown, grey, yellow, or colourless. Its distinctive colour makes it popular among mineral collectors. Itsstreakcan be either pale yellow or brownish-yellow. Vanadinite may betransparent,translucent oropaque,and itslustrecan range fromresinoustoadamantine.Vanadinite isanisotropic,meaning that some of its properties differ when measured along different axes. When measured perpendicular and parallel to its axis of anisotropy, itsrefractive indicesare 2.350 and 2.416 respectively. This gives it abirefringenceof 0.066.[2][3][5]
Vanadinite is very brittle, producing small,conchoidalfragments whenfractured.Its hardness is 3–4 on theMohs scale,about the same as a copper coin. Vanadinite is particularly heavy for a translucent mineral. It has amolar massof 1416.27g/moland itsspecific gravitycan range between 6.6 and 7.2 because of impurities.[3][5][7]
Uses
[edit]Along withcarnotiteandroscoelite,vanadinite is one of the main industrial ores of the elementvanadium,which can be extracted byroastingandsmelting.Vanadinite is also occasionally used as a source of lead. A common process for extracting the vanadium begins with the heating of vanadinite withsalt(NaCl) orsodium carbonate(Na2CO3) at about 850 °C to producesodium vanadate(NaVO3). This is dissolved in water and then treated withammonium chlorideto give an orange-coloured precipitate ofammonium metavanadate.This is then melted to form a crude form ofvanadium pentoxide(V2O5). Reduction of vanadium pentoxide withcalciumgives pure vanadium.[11]
Image gallery
[edit]-
Vanadinite inhexagonalshaped crystals
-
Vanadinite that does not show the characteristic red colour
-
A pile of thousands of crystals, showing their hexagonal shape
-
Vanadinite onbarite,from Mibladene, Upper Moulouya lead district, Midelt, Drâa-Tafilalet Region, Morocco
-
Crystals of vanadinite (var. endlichite), from Touissit, Touissit District, Oujda-Angad Province, Oriental Region, Morocco
-
Crystals of vanadinite andsiderite,from Taouz Er Rachidia Province, Drâa-Tafilalet Region, Morocco
See also
[edit]References
[edit]- ^Warr, L.N. (2021)."IMA–CNMNC approved mineral symbols".Mineralogical Magazine.85(3): 291–320.Bibcode:2021MinM...85..291W.doi:10.1180/mgm.2021.43.S2CID235729616.
- ^abcd"Vanadinite Mineral Data".WebMineral.com.Retrieved9 June2007.
- ^abcdef"Vanadinite".MinDat.org.Retrieved9 June2007.
- ^abAnthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (2000). "Vanadinite".Handbook of Mineralogy(PDF).Vol. IV (Arsenates, Phosphates, Vanadates). Chantilly, VA, US: Mineralogical Society of America.ISBN0962209732.
- ^abcdefTreasures of the Earth: The Minerals and Gemstone Collection – Vanadinite factsheet.Orbis Publishing Ltd. 1995.
- ^J. A. Pérez-Bustamante de Monasterio (1990). "Highlights of Spanish chemistry at the time of the chemical revolution of the 18th century".Fresenius' Journal of Analytical Chemistry.337(2): 225–228.doi:10.1007/BF00322401.S2CID197594307.
- ^abSpencer, Leonard James (1911)..InChisholm, Hugh(ed.).Encyclopædia Britannica.Vol. 27 (11th ed.). Cambridge University Press.
- ^"Vanadinite".Minerals.net.Retrieved26 June2007.
- ^J. Trotter & W. H. Barnes (1958)."The Structure of Vanadinite"(PDF).The Canadian Mineralogist.Retrieved26 June2007.
- ^Dai, Yongshan; Hughes, John M. (1989)."Crystal structure refinements of vanadinite and pyromorphite"(PDF).Can. Mineral.27(2): 189–192.
- ^Donal O'Leary (2000)."Vanadium".University College Cork. Archived fromthe originalon 5 February 2017.Retrieved26 June2007.