Angiogenesis
| Angiogenesis | |
|---|---|
 Angiogenesis following vasculogenesis | |
| Identifiers | |
| MeSH | D000096482 |
| Anatomical terminology | |

Angiogenesisis the physiological process through which newblood vesselsform from pre-existing vessels,[1][2][3]formed in the earlier stage ofvasculogenesis.Angiogenesis continues the growth of thevasculaturemainly by processes of sprouting and splitting, but processes such ascoalescent angiogenesis,[4]vessel elongation and vessel cooption also play a role.[2]Vasculogenesis is theembryonicformation ofendothelialcells frommesodermcell precursors,[5]and fromneovascularization,although discussions are not always precise (especially in older texts). The first vessels in the developingembryoform through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth duringdevelopmentand in disease.[6]
Angiogenesis is a normal and vital process in growth and development, as well as inwound healingand in the formation ofgranulation tissue.However, it is also a fundamental step in the transition oftumorsfrom a benign state to amalignantone, leading to the use ofangiogenesis inhibitorsin the treatment ofcancer.[7]The essential role of angiogenesis in tumor growth was first proposed in 1971 byJudah Folkman,who described tumors as "hot and bloody,"[8]illustrating that, at least for many tumor types, flushperfusionand evenhyperemiaare characteristic.
Types
[edit]Sprouting angiogenesis
[edit]Sprouting angiogenesis was the first identified form of angiogenesis and because of this, it is much more understood than intussusceptive angiogenesis. It occurs in several well-characterized stages. The initial signal comes from tissue areas that are devoid of vasculature. Thehypoxiathat is noted in these areas causes the tissues to demand the presence of nutrients and oxygen that will allow the tissue to carry out metabolic activities. Because of this, parenchymal cells will secrete vascular endothelial growth factor (VEGF-A) which is a proangiogenic growth factor.[9]These biological signals activatereceptorsonendothelial cellspresent in pre-existing blood vessels. Second, the activated endothelial cells, also known astip cells,[10]begin to releaseenzymescalledproteasesthat degrade thebasement membraneto allow endothelial cells to escape from the original (parent) vessel walls. Theendothelial cellsthenproliferateinto the surroundingmatrixand form solid sprouts connecting neighboring vessels. The cells that are proliferating are located behind the tip cells and are known asstalk cells.[10]The proliferation of these cells allows the capillary sprout to grow in length simultaneously.
As sprouts extend toward the source of the angiogenic stimulus, endothelial cells migrate intandem,using adhesion molecules calledintegrins.These sprouts then form loops to become a full-fledged vessellumenas cells migrate to the site of angiogenesis. Sprouting occurs at a rate of several millimeters per day, and enables new vessels to grow across gaps in thevasculature.It is markedly different from splitting angiogenesis because it forms entirely new vessels as opposed to splitting existing vessels.
Intussusceptive angiogenesis
[edit]Intussusceptive angiogenesis,also known assplitting angiogenesis,is the formation of a new blood vessel by splitting an existing blood vessel into two.
Intussusception was first observed inneonatalrats. In this type of vessel formation, the capillary wall extends into thelumento split a single vessel in two. There are four phases of intussusceptive angiogenesis. First, the two opposing capillary walls establish a zone of contact. Second, theendothelialcell junctionsare reorganized and the vesselbilayerisperforatedto allowgrowth factorsand cells to penetrate into the lumen. Third, a core is formed between the 2 new vessels at the zone of contact that is filled withpericytesandmyofibroblasts.These cells begin layingcollagenfibers into the core to provide anextracellular matrixfor growth of the vessel lumen. Finally, the core is fleshed out with no alterations to the basic structure. Intussusception is important because it is a reorganization of existing cells. It allows a vast increase in the number ofcapillarieswithout a corresponding increase in the number ofendothelial cells.This is especially important in embryonic development as there are not enough resources to create a richmicrovasculaturewith new cells every time a new vessel develops.[11]
Coalescent angiogenesis
[edit]Coalescent angiogenesisis a mode of angiogenesis, considered to be the opposite of intussusceptive angiogenesis, where capillaries fuse, or coalesce, to make a larger bloodvessel, thereby increasing blood flow and circulation.[12]Coalescent angiogenesis has extended out of the domain of embryology. It is assumed to play a role in the formation of neovasculature, such as in a tumor.[13]
Physiology
[edit]Mechanical stimulation
[edit]Mechanical stimulation of angiogenesis is not well characterized. There is a significant amount of controversy with regard toshear stressacting on capillaries to cause angiogenesis, although current knowledge suggests that increased muscle contractions may increase angiogenesis.[14]This may be due to an increase in the production ofnitric oxideduring exercise. Nitric oxide results in vasodilation of blood vessels.
Chemical stimulation
[edit]Chemical stimulation of angiogenesis is performed by various angiogenic proteins e.g. integrins and prostaglandins, including severalgrowth factorse.g. VEGF, FGF.
Overview
[edit]| Stimulator | Mechanism |
|---|---|
| FGF | Promotes proliferation & differentiation of endothelial cells, smooth muscle cells, and fibroblasts |
| VEGF | Affects permeability |
| VEGFRandNRP-1 | Integrate survival signals |
| Ang1andAng2 | Stabilize vessels |
| PDGF(BB-homodimer) andPDGFR | recruitsmooth muscle cells |
| TGF-β,endoglinandTGF-β receptors | ↑extracellular matrixproduction |
| CCL2 | Recruitslymphocytesto sites ofinflammation |
| Histamine | |
| IntegrinsαVβ3,αVβ5(?[15]) andα5β1 | Bindmatrix macromoleculesandproteinases |
| VE-cadherinandCD31 | endothelialjunctional molecules |
| ephrin | Determine formation of arteries or veins |
| plasminogen activators | remodelsextracellular matrix,releases and activates growth factors |
| plasminogen activator inhibitor-1 | stabilizes nearby vessels |
| eNOSandCOX-2 | |
| AC133 | regulatesangioblastdifferentiation |
| ID1/ID3 | Regulates endothelialtransdifferentiation |
| Class 3semaphorins | Modulates endothelial cell adhesion, migration, proliferation and apoptosis. Alters vascular permeability[16] |
| Nogo-A | Regulates endothelial cell migration and proliferation.[17]Alters vascular permeability.[18] |
FGF
[edit]Thefibroblast growth factor(FGF) family with its prototype membersFGF-1(acidic FGF) andFGF-2(basic FGF) consists to date of at least 22 known members.[19]Most are single-chain peptides of 16-18 kDa and display high affinity to heparin and heparan sulfate. In general, FGFs stimulate a variety of cellular functions by binding to cell surface FGF-receptors in the presence of heparin proteoglycans. The FGF-receptor family is composed of seven members, and all the receptor proteins are single-chain receptor tyrosine kinases that become activated through autophosphorylation induced by a mechanism of FGF-mediated receptor dimerization. Receptor activation gives rise to a signal transduction cascade that leads to gene activation and diverse biological responses, including cell differentiation, proliferation, and matrix dissolution, thus initiating a process of mitogenic activity critical for the growth of endothelial cells, fibroblasts, and smooth muscle cells. FGF-1, unique among all 22 members of the FGF family, can bind to all seven FGF-receptor subtypes, making it the broadest-acting member of the FGF family, and a potent mitogen for the diverse cell types needed to mount an angiogenic response in damaged (hypoxic) tissues, where upregulation of FGF-receptors occurs.[20]FGF-1 stimulates the proliferation and differentiation of all cell types necessary for building an arterial vessel, including endothelial cells and smooth muscle cells; this factdistinguishes FGF-1 from other pro-angiogenic growth factors,such asvascular endothelial growth factor(VEGF), which primarily drives the formation of new capillaries.[21][22]
Besides FGF-1, one of the most important functions of fibroblast growth factor-2 (FGF-2 orbFGF) is the promotion of endothelial cell proliferation and the physical organization of endothelial cells into tube-like structures, thus promoting angiogenesis. FGF-2 is a more potent angiogenic factor than VEGF or PDGF (platelet-derived growth factor); however, it is less potent than FGF-1. As well as stimulating blood vessel growth, aFGF (FGF-1) and bFGF (FGF-2) are important players in wound healing. They stimulate the proliferation of fibroblasts and endothelial cells that give rise to angiogenesis and developing granulation tissue; both increase blood supply and fill up a wound space/cavity early in the wound-healing process.
VEGF
[edit]Vascular endothelial growth factor(VEGF) has been demonstrated to be a major contributor to angiogenesis, increasing the number of capillaries in a given network. Initialin vitrostudies demonstrated bovine capillary endothelial cells will proliferate and show signs of tube structures upon stimulation by VEGF andbFGF,although the results were more pronounced with VEGF.[23]Upregulation of VEGF is a major component of the physiological response to exercise and its role in angiogenesis is suspected to be a possible treatment in vascular injuries.[24][25][26][27]In vitrostudies clearly demonstrate that VEGF is a potent stimulator of angiogenesis because, in the presence of this growth factor, plated endothelial cells will proliferate and migrate, eventually forming tube structures resembling capillaries.[14] VEGF causes a massive signaling cascade inendothelialcells. Binding to VEGF receptor-2 (VEGFR-2) starts a tyrosine kinase signaling cascade that stimulates the production of factors that variously stimulate vessel permeability (eNOS, producing NO), proliferation/survival (bFGF), migration (ICAMs/VCAMs/MMPs) and finally differentiation into mature blood vessels. Mechanically, VEGF is upregulated with muscle contractions as a result of increased blood flow to affected areas. The increased flow also causes a large increase in themRNAproduction of VEGF receptors 1 and 2. The increase in receptor production means muscle contractions could cause upregulation of the signaling cascade relating to angiogenesis. As part of the angiogenic signaling cascade, NO is widely considered to be a major contributor to the angiogenic response because inhibition of NO significantly reduces the effects of angiogenic growth factors. However, inhibition of NO during exercise does not inhibit angiogenesis, indicating there are other factors involved in the angiogenic response.[14]
Angiopoietins
[edit]Theangiopoietins,Ang1 and Ang2, are required for the formation of mature blood vessels, as demonstrated by mouseknock outstudies.[28]Ang1andAng2are protein growth factors which act by binding their receptors,Tie-1andTie-2;while this is somewhat controversial, it seems that cell signals are transmitted mostly byTie-2;though some papers show physiologic signaling viaTie-1as well. These receptors aretyrosine kinases.Thus, they can initiatecell signalingwhen ligand binding causes a dimerization that initiatesphosphorylationon key tyrosines.
MMP
[edit]Another major contributor to angiogenesis ismatrix metalloproteinase(MMP). MMPs help degrade the proteins that keep the vessel walls solid. Thisproteolysisallows theendothelial cellsto escape into the interstitial matrix as seen in sprouting angiogenesis. Inhibition of MMPs prevents the formation of newcapillaries.[29]Theseenzymesare highly regulated during the vessel formation process because destruction of theextracellular matrixwould decrease the integrity of the microvasculature.[14]
Dll4
[edit]Delta-like ligand 4(Dll4) is a protein with a negative regulatory effect on angiogenesis.[30][31]Dll4 is a transmembrane ligand, for thenotch family of receptors.There have been many studies conducted that have served to determine consequences of the Delta-like Ligand 4. One study in particular evaluated the effects of Dll4 on tumor vascularity and growth.[32]In order for a tumor to grow and develop, it must have the proper vasculature. The VEGF pathway is vital to the development of vasculature that in turn, helps the tumors to grow. The combined blockade of VEGF and Dll4 results in the inhibition of tumor progression and angiogenesis throughout the tumor. This is due to the hindrance of signaling in endothelial cell signaling which cuts off the proliferation and sprouting of these endothelial cells. With this inhibition, the cells do not uncontrollably grow, therefore, the cancer is stopped at this point. if the blockade, however, were to be lifted, the cells would begin their proliferation once again.[33]
Class 3 semaphorins
[edit]Class 3 semaphorins(SEMA3s) regulate angiogenesis by modulatingendothelial celladhesion, migration, proliferation, survival and the recruitment ofpericytes.[16]Furthermore,semaphorinscan interfere with VEGF-mediated angiogenesis since both SEMA3s andVEGF-Acompete forneuropilinreceptor binding at endothelial cells.[34][35]The relative expression levels of SEMA3s and VEGF-A may therefore be important for angiogenesis.[16]
Chemical inhibition
[edit]Anangiogenesis inhibitorcan be endogenous or come from outside asdrugor adietary component.
Application in medicine
[edit]Angiogenesis as a therapeutic target
[edit]Angiogenesis may be a target for combating diseases such asheart diseasecharacterized by either poor vascularisation or abnormal vasculature.[36]Application of specific compounds that may inhibit or induce the creation of newblood vesselsin the body may help combat such diseases. The presence of blood vessels where there should be none may affect the mechanical properties of a tissue, increasing the likelihood of failure. The absence of blood vessels in a repairing or otherwise metabolically active tissue may inhibit repair or other essential functions. Several diseases, such asischemic chronic wounds,are the result of failure or insufficient blood vessel formation and may be treated by a local expansion of blood vessels, thus bringing new nutrients to the site, facilitating repair. Other diseases, such as age-relatedmacular degeneration,may be created by a local expansion of blood vessels, interfering with normal physiological processes.
The modern clinical application of the principle of angiogenesis can be divided into two main areas: anti-angiogenic therapies, which angiogenic research began with, and pro-angiogenic therapies. Whereas anti-angiogenic therapies are being employed to fight cancer and malignancies,[37][38]which require an abundance ofoxygenand nutrients to proliferate, pro-angiogenic therapies are being explored as options to treatcardiovascular diseases,the number one cause of death in theWestern world.One of the first applications of pro-angiogenic methods in humans was a German trial using fibroblast growth factor 1 (FGF-1) for the treatment of coronary artery disease.[21][39][40]
Regarding themechanism of action,pro-angiogenic methods can be differentiated into three main categories:gene therapy,targeting genes of interest for amplification or inhibition;protein replacement therapy,which primarily manipulates angiogenic growth factors likeFGF-1orvascular endothelial growth factor,VEGF; and cell-based therapies, which involve the implantation of specific cell types.
There are still serious, unsolved problems related to gene therapy. Difficulties include effective integration of the therapeutic genes into the genome of target cells, reducing the risk of an undesired immune response, potential toxicity,immunogenicity,inflammatory responses, andoncogenesisrelated to the viral vectors used in implanting genes and the sheer complexity of the genetic basis of angiogenesis. The most commonly occurring disorders in humans, such as heart disease, high blood pressure, diabetes andAlzheimer's disease,are most likely caused by the combined effects of variations in many genes, and, thus, injecting a single gene may not be significantly beneficial in such diseases.[citation needed]
By contrast, pro-angiogenic protein therapy uses well-defined, precisely structured proteins, with previously defined optimal doses of the individual protein for disease states, and with well-known biological effects.[1]On the other hand, an obstacle of protein therapy is the mode of delivery. Oral, intravenous, intra-arterial, or intramuscular routes of protein administration are not always as effective, as the therapeutic protein may be metabolized or cleared before it can enter the target tissue. Cell-based pro-angiogenic therapies are still early stages of research, with many open questions regarding best cell types and dosages to use.
Tumor angiogenesis
[edit]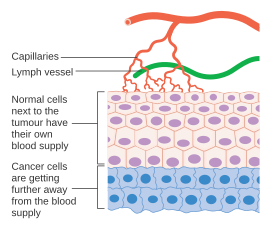
Cancer cells are cells that have lost their ability to divide in a controlled fashion. Amalignant tumorconsists of a population of rapidly dividing and growing cancer cells that progressively accruesmutations.However, tumors need a dedicated blood supply to provide the oxygen and other essential nutrients they require in order to grow beyond a certain size (generally 1–2 mm3).[41][42]
Tumors induce blood vessel growth (angiogenesis) by secreting various growth factors (e.g.VEGF) and proteins. Growth factors such asbFGFandVEGFcan induce capillary growth into the tumor, which some researchers suspect supply required nutrients, allowing for tumor expansion. Unlike normal blood vessels, tumor blood vessels are dilated with an irregular shape.[43]Other clinicians believe angiogenesis really serves as a waste pathway, taking away the biological end products secreted by rapidly dividing cancer cells. In either case, angiogenesis is a necessary and required step for transition from a small harmless cluster of cells, often said to be about the size of the metal ball at the end of a ball-point pen, to a large tumor. Angiogenesis is also required for the spread of a tumor, ormetastasis.[7]Single cancer cells can break away from an established solid tumor, enter the blood vessel, and be carried to a distant site, where they can implant and begin the growth of a secondary tumor. Evidence now suggests the blood vessel in a given solid tumor may, in fact, be mosaic vessels, composed ofendothelial cellsand tumor cells.[7]This mosaicity allows for substantial shedding of tumor cells into the vasculature, possibly contributing to the appearance ofcirculating tumor cellsin the peripheral blood of patients with malignancies.[44]The subsequent growth of such metastases will also require a supply of nutrients andoxygenand a waste disposal pathway.
Endothelial cells have long been considered genetically more stable than cancer cells. This genomic stability confers an advantage to targeting endothelial cells using antiangiogenic therapy, compared tochemotherapydirected at cancer cells, which rapidly mutate and acquiredrug resistanceto treatment. For this reason,endothelial cellsare thought to be an ideal target for therapies directed against them.[45]
Formation of tumor blood vessels
[edit]The mechanism of blood vessel formation by angiogenesis is initiated by the spontaneous dividing of tumor cells due to a mutation. Angiogenic stimulators are then released by the tumor cells. These then travel to already established, nearby blood vessels and activates their endothelial cell receptors. This induces a release ofproteolyticenzymes from the vasculature. These enzymes target a particular point on the blood vessel and cause a pore to form. This is the point where the new blood vessel will grow from. The reason tumour cells need a blood supply is because they cannot grow any more than 2-3 millimeters in diameter without an established blood supply which is equivalent to about 50-100 cells.[46]Certain studies have indicated that vessels formed inside the tumor tissue are of higher irregularity and bigger in size, which is as well associated with poorer prognosis.[47][48]
Angiogenesis for cardiovascular disease
[edit]Angiogenesis represents an excellent therapeutic target for the treatment of cardiovascular disease. It is a potent, physiological process that underlies the natural manner in which our bodies respond to a diminution of blood supply to vital organs, namelyneoangiogenesis:the production of new collateral vessels to overcome the ischemic insult.[21]A large number of preclinical studies have been performed with protein-, gene- and cell-based therapies in animal models of cardiac ischemia, as well as models of peripheral artery disease. Reproducible and credible successes in these early animal studies led to high enthusiasm that this new therapeutic approach could be rapidly translated to a clinical benefit for millions of patients in the Western world with these disorders. A decade of clinical testing both gene- and protein-based therapies designed to stimulate angiogenesis in underperfused tissues and organs, however, has led from one disappointment to another. Although all of these preclinical readouts, which offered great promise for the transition of angiogenesis therapy from animals to humans, were in one fashion or another, incorporated into early stage clinical trials, the FDA has, to date (2007), insisted that the primary endpoint for approval of an angiogenic agent must be an improvement in exercise performance of treated patients.[49]
These failures suggested that either these are the wrong molecular targets to induce neovascularization, that they can only be effectively used if formulated and administered correctly, or that theirpresentationin the context of the overall cellular microenvironment may play a vital role in their utility. It may be necessary to present these proteins in a way that mimics natural signaling events, including theconcentration,spatialandtemporalprofiles, and their simultaneous or sequential presentation with other appropriate factors.[50]
Exercise
[edit]Angiogenesis is generally associated withaerobic exerciseandendurance exercise.Whilearteriogenesisproduces network changes that allow for a large increase in the amount of total flow in a network, angiogenesis causes changes that allow for greater nutrient delivery over a long period of time. Capillaries are designed to provide maximum nutrient delivery efficiency, so an increase in the number of capillaries allows the network to deliver more nutrients in the same amount of time. A greater number of capillaries also allows for greater oxygen exchange in the network. This is vitally important to endurance training, because it allows a person to continue training for an extended period of time. However, no experimental evidence suggests that increased capillarity is required in endurance exercise to increase the maximum oxygen delivery.[14]
Macular degeneration
[edit]Overexpression of VEGF causes increased permeability in blood vessels in addition to stimulating angiogenesis. In wetmacular degeneration,VEGF causes proliferation of capillaries into the retina. Since the increase in angiogenesis also causesedema,blood and other retinal fluids leak into theretina,causing loss of vision. Anti-angiogenic drugs targeting the VEGF pathways are now used successfully to treat this type of macular degeneration
Tissue engineered constructs
[edit]Angiogenesis of vessels from the host body into an implanted tissue engineered constructs is essential. Successful integration is often dependent on thorough vascularisation of the construct as it provides oxygen and nutrients and prevents necrosis in the central areas of the implant.[51]PDGF has been shown to stabilize vascularisation in collagen-glycosaminoglycanscaffolds.[52]
History
[edit]The first report of angiogenesis can be traced back to the bookA treatise on the blood, inflammation, and gun-shot woundspublished in 1794, where Scottish anatomistJohn Hunter's research findings were compiled. In his study, Hunter observed the growth process of new blood vessels in rabbits. However, he did not coin the term "Angiogenesis," which is now widely used by scholars. Hunter also erroneously attributed the growth process of new blood vessels to the effect of an innate vital principle within the blood. The term "angiogenesis" is believed to have emerged not until the 1900s. The inception of modern angiogenesis research is marked by Judah Folkman's report on the pivotal role of angiogenesis in tumor growth.[8][53][54]
Quantification
[edit]Quantifying vasculature parameters such as microvascular density has various complications due to preferential staining or limited representation of tissues by histological sections. Recent research has shown complete 3D reconstruction of tumor vascular structure and quantification of vessel structures in whole tumors in animal models.[55]
See also
[edit]References
[edit]- ^abSantulli G, ed. (2013).Angiogenesis insights from a systematic overview.New York: Nova Science.ISBN978-1-62618-114-4.
- ^abDudley AC, Griffioen AW (August 2023)."Pathological angiogenesis: mechanisms and therapeutic strategies".Angiogenesis.26(3): 313–347.doi:10.1007/s10456-023-09876-7.PMC10105163.PMID37060495.
- ^Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (July 2014)."Type-2 pericytes participate in normal and tumoral angiogenesis".American Journal of Physiology. Cell Physiology.307(1): C25–C38.doi:10.1152/ajpcell.00084.2014.PMC4080181.PMID24788248.
- ^Nitzsche B, Rong WW, Goede A, Hoffmann B, Scarpa F, Kuebler WM, et al. (February 2022)."Coalescent angiogenesis-evidence for a novel concept of vascular network maturation".Angiogenesis.25(1): 35–45.doi:10.1007/s10456-021-09824-3.PMC8669669.PMID34905124.
- ^Risau W, Flamme I (1995). "Vasculogenesis".Annual Review of Cell and Developmental Biology.11:73–91.doi:10.1146/annurev.cb.11.110195.000445.PMID8689573.
- ^Flamme I, Frölich T, Risau W (November 1997). "Molecular mechanisms of vasculogenesis and embryonic angiogenesis".Journal of Cellular Physiology.173(2): 206–210.doi:10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C.PMID9365523.S2CID36723610.
- ^abcMilosevic V, Edelmann RJ, Fosse JH, Östman A, Akslen LA (2022). "Molecular Phenotypes of Endothelial Cells in Malignant Tumors". In Akslen LA, Watnick RS (eds.).Biomarkers of the Tumor Microenvironment.Cham: Springer International Publishing. pp. 31–52.doi:10.1007/978-3-030-98950-7_3.ISBN978-3-030-98950-7.
- ^abPenn JS (11 March 2008).Retinal and Choroidal Angiogenesis.Springer. pp. 119–.ISBN978-1-4020-6779-2.Retrieved26 June2010.
- ^Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Overview of Angiogenesis. Available from:https://www.ncbi.nlm.nih.gov/books/NBK53238/
- ^abWeavers H, Skaer H (July 2014)."Tip cells: master regulators of tubulogenesis?".Seminars in Cell & Developmental Biology.31(100): 91–99.doi:10.1016/j.semcdb.2014.04.009.PMC4071413.PMID24721475.
- ^Burri PH, Hlushchuk R, Djonov V (November 2004)."Intussusceptive angiogenesis: its emergence, its characteristics, and its significance".Developmental Dynamics.231(3): 474–488.doi:10.1002/dvdy.20184.PMID15376313.S2CID35018922.
- ^Nitzsche B, Rong WW, Goede A, Hoffmann B, Scarpa F, Kuebler WM, et al. (February 2022)."Coalescent angiogenesis-evidence for a novel concept of vascular network maturation".Angiogenesis.25(1): 35–45.doi:10.1007/s10456-021-09824-3.PMC8669669.PMID34905124.
- ^Pezzella F, Kerbel RS (February 2022)."On coalescent angiogenesis and the remarkable flexibility of blood vessels".Angiogenesis.25(1): 1–3.doi:10.1007/s10456-021-09825-2.PMID34993716.S2CID254188870.
- ^abcdePrior BM, Yang HT, Terjung RL (September 2004). "What makes vessels grow with exercise training?".Journal of Applied Physiology.97(3): 1119–1128.doi:10.1152/japplphysiol.00035.2004.PMID15333630.
- ^Perhaps an inhibitor of angiogenesis:Sheppard D (October 2002)."Endothelial integrins and angiogenesis: not so simple anymore".The Journal of Clinical Investigation.110(7): 913–914.doi:10.1172/JCI16713.PMC151161.PMID12370267.
- ^abcMecollari V, Nieuwenhuis B, Verhaagen J (2014)."A perspective on the role of class III semaphorin signaling in central nervous system trauma".Frontiers in Cellular Neuroscience.8:328.doi:10.3389/fncel.2014.00328.PMC4209881.PMID25386118.
- ^Rust R, Grönnert L, Gantner C, Enzler A, Mulders G, Weber RZ, et al. (July 2019)."Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke".Proceedings of the National Academy of Sciences of the United States of America.116(28): 14270–14279.Bibcode:2019PNAS..11614270R.doi:10.1073/pnas.1905309116.PMC6628809.PMID31235580.
- ^Rust R, Weber RZ, Grönnert L, Mulders G, Maurer MA, Hofer AS, et al. (December 2019)."Anti-Nogo-A antibodies prevent vascular leakage and act as pro-angiogenic factors following stroke".Scientific Reports.9(1): 20040.Bibcode:2019NatSR...920040R.doi:10.1038/s41598-019-56634-1.PMC6934709.PMID31882970.
- ^Ornitz DM, Itoh N (2001)."Fibroblast growth factors".Genome Biology.2(3): REVIEWS3005.doi:10.1186/gb-2001-2-3-reviews3005.PMC138918.PMID11276432.
- ^Blaber M, DiSalvo J, Thomas KA (February 1996). "X-ray crystal structure of human acidic fibroblast growth factor".Biochemistry.35(7): 2086–2094.CiteSeerX10.1.1.660.7607.doi:10.1021/bi9521755.PMID8652550.
- ^abcStegmann TJ (December 1998). "FGF-1: a human growth factor in the induction of neoangiogenesis".Expert Opinion on Investigational Drugs.7(12): 2011–2015.doi:10.1517/13543784.7.12.2011.PMID15991943.
- ^Khurana R, Simons M (April 2003). "Insights from angiogenesis trials using fibroblast growth factor for advanced arteriosclerotic disease".Trends in Cardiovascular Medicine.13(3): 116–122.doi:10.1016/S1050-1738(02)00259-1.PMID12691676.
- ^Goto F, Goto K, Weindel K, Folkman J (November 1993). "Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels".Laboratory Investigation; A Journal of Technical Methods and Pathology.69(5): 508–517.PMID8246443.
- ^Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y (December 2004)."Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke".Current Neurovascular Research.1(5): 411–420.doi:10.2174/1567202043361875.PMID16181089.S2CID22015361.Archived fromthe originalon April 19, 2012.
- ^Gavin TP, Robinson CB, Yeager RC, England JA, Nifong LW, Hickner RC (January 2004). "Angiogenic growth factor response to acute systemic exercise in human skeletal muscle".Journal of Applied Physiology.96(1): 19–24.doi:10.1152/japplphysiol.00748.2003.PMID12949011.S2CID12750224.
- ^Kraus RM, Stallings HW, Yeager RC, Gavin TP (April 2004). "Circulating plasma VEGF response to exercise in sedentary and endurance-trained men".Journal of Applied Physiology.96(4): 1445–1450.doi:10.1152/japplphysiol.01031.2003.PMID14660505.S2CID21090407.
- ^Lloyd PG, Prior BM, Yang HT, Terjung RL (May 2003). "Angiogenic growth factor expression in rat skeletal muscle in response to exercise training".American Journal of Physiology. Heart and Circulatory Physiology.284(5): H1668–H1678.doi:10.1152/ajpheart.00743.2002.PMID12543634.
- ^Thurston G (October 2003). "Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis".Cell and Tissue Research.314(1): 61–68.doi:10.1007/s00441-003-0749-6.PMID12915980.S2CID2529783.
- ^Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, et al. (October 2000). "Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle".American Journal of Physiology. Heart and Circulatory Physiology.279(4): H1540–H1547.doi:10.1152/ajpheart.2000.279.4.H1540.PMID11009439.S2CID2543076.
- ^Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ (February 2007)."Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting".Proceedings of the National Academy of Sciences of the United States of America.104(9): 3219–3224.Bibcode:2007PNAS..104.3219L.doi:10.1073/pnas.0611206104.PMC1805530.PMID17296940.
- ^Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. (February 2007). "Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis".Nature.445(7129): 776–780.Bibcode:2007Natur.445..776H.doi:10.1038/nature05571.PMID17259973.S2CID4407198.
- ^Segarra M, Williams CK, Sierra Mde L, Bernardo M, McCormick PJ, Maric D, et al. (September 2008)."Dll4 activation of Notch signaling reduces tumor vascularity and inhibits tumor growth".Blood.112(5): 1904–11.doi:10.1182/blood-2007-11-126045.PMC2518892.PMID18577711.
- ^Lee D, Kim D, Choi YB, Kang K, Sung ES, Ahn JH, et al. (July 2016)."Simultaneous blockade of VEGF and Dll4 by HD105, a bispecific antibody, inhibits tumor progression and angiogenesis".mAbs.8(5): 892–904.doi:10.1080/19420862.2016.1171432.PMC4968104.PMID27049350.
- ^Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (March 1998)."Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor".Cell.92(6): 735–745.doi:10.1016/s0092-8674(00)81402-6.PMID9529250.S2CID547080.
- ^Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC (August 2011)."VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation".Molecular Biology of the Cell.22(15): 2766–2776.doi:10.1091/mbc.E09-12-1061.PMC3145551.PMID21653826.
- ^Ferrara N, Kerbel RS (December 2005). "Angiogenesis as a therapeutic target".Nature.438(7070): 967–974.Bibcode:2005Natur.438..967F.doi:10.1038/nature04483.PMID16355214.S2CID1183610.
- ^Folkman J, Klagsbrun M (January 1987). "Angiogenic factors".Science.235(4787): 442–447.Bibcode:1987Sci...235..442F.doi:10.1126/science.2432664.PMID2432664.
- ^Folkman J (September 1996). "Fighting cancer by attacking its blood supply".Scientific American.275(3): 150–154.Bibcode:1996SciAm.275c.150F.doi:10.1038/scientificamerican0996-150.PMID8701285.
- ^Stegmann TJ, Hoppert T, Schneider A, Gemeinhardt S, Köcher M, Ibing R, Strupp G (September 2000). "[Induction of myocardial neoangiogenesis by human growth factors. A new therapeutic approach in coronary heart disease]".Herz(in German).25(6): 589–599.doi:10.1007/PL00001972.PMID11076317.S2CID21240045.
- ^Folkman J (February 1998)."Angiogenic therapy of the human heart".Circulation.97(7): 628–629.doi:10.1161/01.CIR.97.7.628.PMID9495294.
- ^McDougall SR, Anderson AR, Chaplain MA (August 2006). "Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies".Journal of Theoretical Biology.241(3): 564–589.Bibcode:2006JThBi.241..564M.doi:10.1016/j.jtbi.2005.12.022.PMID16487543.
- ^Spill F, Guerrero P, Alarcon T, Maini PK, Byrne HM (February 2015)."Mesoscopic and continuum modelling of angiogenesis".Journal of Mathematical Biology.70(3): 485–532.arXiv:1401.5701.doi:10.1007/s00285-014-0771-1.PMC5320864.PMID24615007.
- ^Gonzalez-Perez RR, Rueda BR (2013).Tumor angiogenesis regulators(first ed.). Boca Raton: Taylor & Francis. p. 347.ISBN978-1-4665-8097-8.Retrieved2 October2014.
- ^Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. (October 2004)."Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases".Clinical Cancer Research.10(20): 6897–6904.doi:10.1158/1078-0432.CCR-04-0378.PMID15501967.
- ^Bagri A, Kouros-Mehr H, Leong KG, Plowman GD (March 2010). "Use of anti-VEGF adjuvant therapy in cancer: challenges and rationale".Trends in Molecular Medicine.16(3): 122–132.doi:10.1016/j.molmed.2010.01.004.PMID20189876.
- ^Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (September 2006)."Angiogenesis in cancer".Vascular Health and Risk Management.2(3): 213–219.doi:10.2147/vhrm.2006.2.3.213.PMC1993983.PMID17326328.
- ^Milosevic V, Edelmann RJ, Winge I, Strell C, Mezheyeuski A, Knutsvik G, et al. (July 2023)."Vessel size as a marker of survival in estrogen receptor positive breast cancer".Breast Cancer Research and Treatment.200(2): 293–304.doi:10.1007/s10549-023-06974-4.PMC10241708.PMID37222874.
- ^Mikalsen LT, Dhakal HP, Bruland ØS, Naume B, Borgen E, Nesland JM, Olsen DR (2013-10-11). Aoki I (ed.)."The clinical impact of mean vessel size and solidity in breast carcinoma patients".PLOS ONE.8(10): e75954.Bibcode:2013PLoSO...875954M.doi:10.1371/journal.pone.0075954.PMC3795733.PMID24146798.
- ^Hariawala MD, Sellke FW (June 1997)."Angiogenesis and the heart: therapeutic implications".Journal of the Royal Society of Medicine.90(6): 307–311.doi:10.1177/014107689709000604.PMC1296305.PMID9227376.
- ^Cao L, Mooney DJ (November 2007)."Spatiotemporal control over growth factor signaling for therapeutic neovascularization".Advanced Drug Delivery Reviews.59(13): 1340–1350.doi:10.1016/j.addr.2007.08.012.PMC2581871.PMID17868951.
- ^Rouwkema J, Khademhosseini A (September 2016)."Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks".Trends in Biotechnology.34(9): 733–745.doi:10.1016/j.tibtech.2016.03.002.PMID27032730.
- ^do Amaral RJ, Cavanagh B, O'Brien FJ, Kearney CJ (February 2019)."Platelet-derived growth factor stabilises vascularisation in collagen-glycosaminoglycan scaffolds in vitro".Journal of Tissue Engineering and Regenerative Medicine.13(2): 261–273.doi:10.1002/term.2789.PMID30554484.S2CID58767660.
- ^Lenzi P, Bocci G, Natale G (April 2016). "John Hunter and the origin of the term" angiogenesis "".Angiogenesis.19(2). Springer Science and Business Media LLC: 255–256.doi:10.1007/s10456-016-9496-7.hdl:11568/795270.PMID26842740.S2CID254189385.
- ^Adair TH, Montani JP (2010)."History".Angiogenesis.Morgan & Claypool Life Sciences.doi:10.4199/C00017ED1V01Y201009ISP009(inactive 31 January 2024).PMID21452444.Retrieved2023-07-20.
{{cite book}}:CS1 maint: DOI inactive as of January 2024 (link) - ^Chien CC, Kempson IM, Wang CL, Chen HH, Hwu Y, Chen NY, et al. (May–June 2013). "Complete microscale profiling of tumor microangiogenesis: a microradiological methodology reveals fundamental aspects of tumor angiogenesis and yields an array of quantitative parameters for its characterization".Biotechnology Advances.31(3): 396–401.doi:10.1016/j.biotechadv.2011.12.001.PMID22193280.
